Physical chemistry studies macroscopic and microscopic aspects in chemical systems. The key to understanding this science lies in the 'physics' descriptor. It implies that this science examines the forces that act on materials' physical properties. But, that's just the physical chemistry iceberg tip; you've much more to know about this fascinating chemistry branch.

A Background on Physical Chemistry
As a child, Wilhelm Ostwald was interested in science, but he studied philosophy, art and humanities at university. He completed his studies in 1875. After graduation, he took an unpaid position as a laboratory assistant, working under the chemist Carl Schmidt. There, he spent his time learning about inorganic chemistry, and chemical reaction rates.
We might say that science captivated him. Still in his unpaid position, he advanced ever deeper, questioning chemicals' affinity and reactivity. He developed three-dimensional models to explain these and other phenomena. He also investigated electrochemistry, chemical dynamics, and mass action.
Wilhelm Ostwald published the magazine's first edition in 1877.
Svante August Arrhenius, and Jacobus Henricus van 't Hoff collaborated.
Wilhelm Ostwald is often hailed as a father of physical chemistry. If anything, he would consider himself a reluctant one, sidetracked from his original pursuits.
Notably, he returned to art and philosophy and made his mark in politics after his academic tenure. Still, for roughly 30 years, science kept its grip on him, leading him to establish many firsts in the physical chemistry field.
Physical Chemistry's Path to Becoming a Science
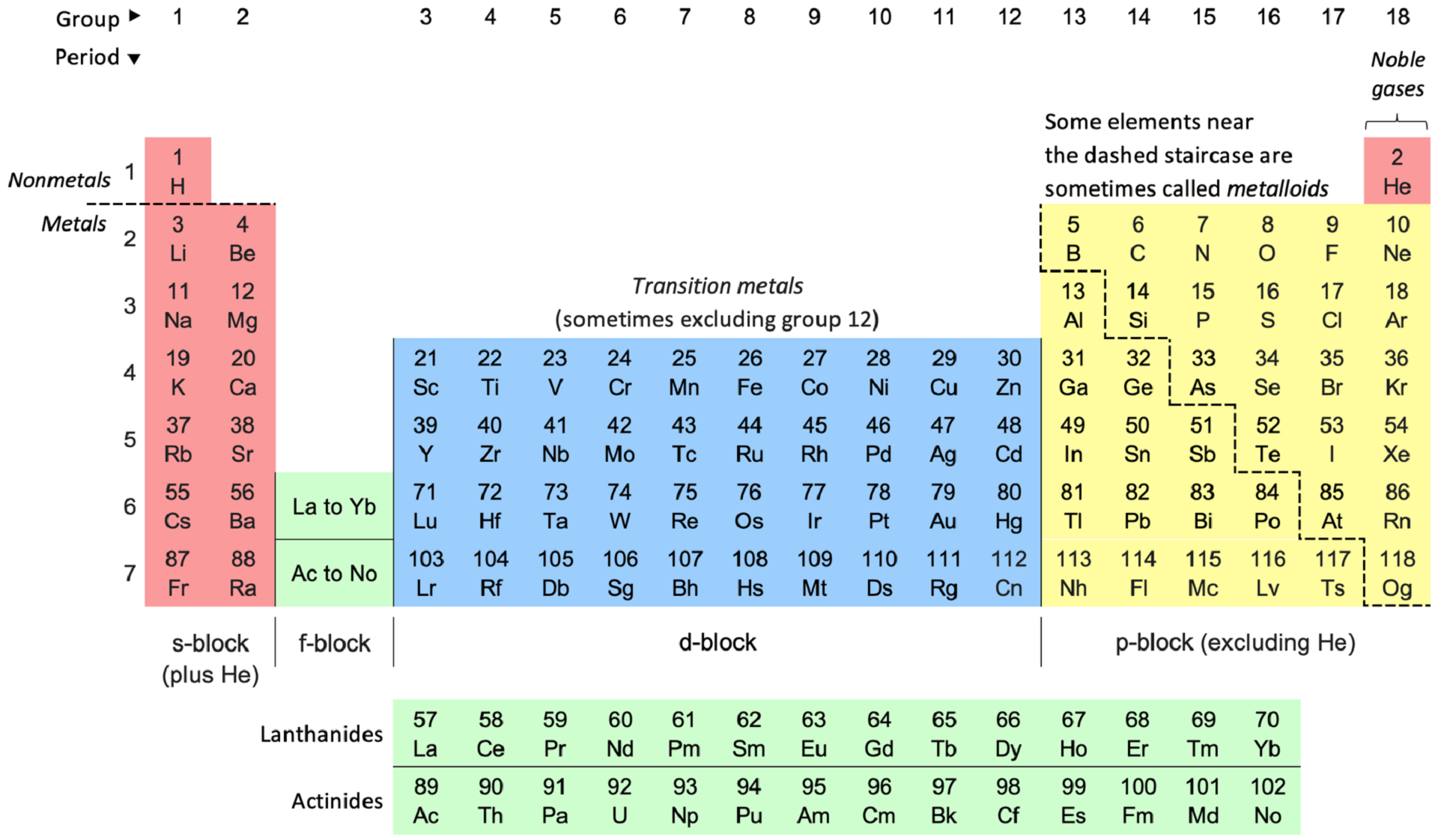
Russian polymath Mikhail Lomonosov established the concept of physical chemistry in 1752, during a lecture. The theory lay untouched for over a century. But then, in those early days, chemistry study was not unified, nor did it have a specified trajectory. That path emerged only when Dmitri Mendeleev established the Periodic Table in 1871.
By that time, interest was growing in the physical properties of chemical action. By 1880, work had begun on chemical thermodynamics and kinetics, among other phenomena.
and description
Ostwald's work on these concepts in that era did much to advance this science. Over his roughly 30-year academic career, he contributed more than 500 research papers and 45 or more books.
The Magazine of Physical Chemistry's timely publication opened the floodgates, cementing physical chemistry as a distinct sub-discipline of chemistry study.
Soon, this publication featured articles about related chemistry discoveries, including analytical chemistry theories. The journal is still in print today, serving as a forum and information source for physical chemistry students and researchers alike.

Concepts in Physical Chemistry
So-called 'wet' chemistry, the kind that uses liquids to provoke reactions, is the type of chemistry we're most familiar with. We can quantify those reactions: solutions change colours and states, for instance.
By contrast, physical chemistry interests itself with what we cannot see - and, often, by what's difficult to measure. For example, what happens to chemical bonds when subjected to heat? How do atoms move when a catalyst - an element that provokes a reaction, is introduced?

These are the concepts of physical chemistry studies. Though one could hardly say this science has 'main' areas, these five are leading concepts in physical chemistry.
Thermodynamics
As its name implies, thermodynamics concerns itself with heat, temperature, and work. Particularly in their relationship to entropy and energy, as well as matter's physical properties. The study of thermodynamics has wide-ranging applications, from physical chemistry and mechanical engineering to meteorology.
The relation of energy with chemical reactions or a physical change of state.
In simple terms, when a chemical reaction takes place, it generates some measure of energy (and heat). The same is true when heat is applied. Imagine boiling water, for instance, which causes it to change from liquid to vapour. Researchers study the spontaneity of those transformations in different conditions.
Chemical Kinetics
Thermodynamics concerns itself with the direction reactions occur in but does not concern itself with the rate of chemical reactions. That is chemical kinetics' purview. This concept investigates how experimental conditions impact chemical reactions.
These studies result in an understanding of transitions' states and reactions' mechanisms. From that data, scientists can build mathematical models to describe chemical reactions and their characteristics.
Chemical Equilibrium
Achieving chemical equilibrium means arriving at a state when a reactant's concentration equals that of a product's. In that state, no further change will happen, even over time. We prove chemical equilibrium when the forward reaction happens at the same rate as the reverse reaction.
1. The conversion of reactants to products.
2. The conversion of products to reactants.
Chemical equilibrium is when these reactions happen at the same time. Its formula is:
aA + bB ⇌ cC + dD
Statistical Mechanics
This mathematical framework applies statistics and probability theory to accumulated microscopic agents. This concept has a direct link to thermodynamics, as it emerged from classical thermodynamic studies.
Thermodynamics focuses on equilibrium; statistical mechanics revolves around modelling the speed of irreversible processes that imbalances provoke.
The tool for predicting how systems behave as they obey the detailed balance principle.
Detailed balance traces back to kinetic and thermodynamic concepts. Understanding these interwoven links is your key explaining to general chemistry concepts, including those present in biochemistry studies.
Quantum Mechanics
This theory describes natural phenomena at the subatomic level. It forms the foundation of quantum physical studies, and explains what classical physics cannot. This explanation is enough to see its relation to studies in physical chemistry.
How Does Physical Chemistry Relate to Other Chemistry Branches?
Studying chemistry is a lot like peeling an onion. Each sub-discipline ties in with, or relies on, the ones above and below. We might say that physical chemistry helps explain all other chemistry studies.
studies organic compounds' structures, properties, and reactions.
explains molecular stability, reaction mechanisms, reaction factors
We can define the relationship between physical chemistry and other types of chemistry, too.
focuses on chemicals' identification, separation, and quantification.
delivers the techniques and equipment to define analytical focus.
Inorganic chemistry is another area where physical chemistry plays an outsized role. This branch of science strives to understand the behaviour and properties of inorganic compounds. This effort would be impossible without first understanding physical chemistry concepts.
Likewise, biochemistry is the study of living organisms' chemical processes. Physical chemistry concepts, such as kinetics and thermodynamics, help define and clarify biochemical processes and reactions.
Let's reflect, for a moment, on physical chemistry's relatively late entry into the chemistry study spectrum. As so many of this branch's aspects feature in 'legacy' chemistry studies, we could say physical chemistry has been a thing all along. It just didn't get its own definition until much later.

What Are Examples of Physical Chemistry?
Explaining science is a lot of fun for minds with that orientation. But, what does it all mean for the practical world? These are some of the most obvious results of applying these principles:
New materials and substances
Medicines and pharmacology
Nanotechnology
Carbon fibre is an excellent example of physical chemistry at work. First produced in 1860 for use in light bulbs, they were too unreliable for any practical purpose.
Lightweight carbon fibre materials find usage in aviation and other high-temperature applications. Everything, from race cars to protective clothing, features carbon fibres.
If you've ever taken a paracetamol tablet or other medicine, thank physical chemistry for its safety. Their research covers everything from the medicine's bioavailability (how much of it the body absorbs), to how stable they are and how quickly they dissolve.
Of late, the media has made much of nanotechnology, particularly where semiconductors are concerned. Here again, physical chemistry is at the forefront of discovery. Were it not for our understanding of nanoparticles and their properties, we could not predict their behaviour under various conditions.
That would mean the end of artificial intelligence potential, supercomputing, and (pretty much) any further electronic advancements.
What Do Physical Chemists Do?

Much of physical chemists' work revolves around research. Whether working in industrial environments or academic facilities, their focus is on discovering and understanding chemical processes. They may work on improving products or creating new materials and substances.
Unless one becomes a teacher, physical chemistry is not a public-facing field. The same is true for anyone researching organic chemistry.
However, even those professionals have a bit more of a chance to work with the public.
Short of dedicating your career to academic research, you're likely to be a chemistry jack-of-all-trades rather than a specialist in your field.
Still, if you're keen to work in environmental research, energy production, or formulating new materials, physical chemistry studies will help you reach your goals.
So will a Superprof physics tutor. Physical chemistry is one of the most difficult subspecialties to grasp. A few sessions with a private tutor will give you a better understanding of these and other chemistry concepts.
Summarise with AI:















