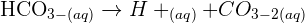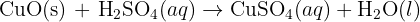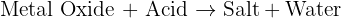A photon interacts with a ground state electron in a hydrogen atom and is absorbed.
I have all of the answers except this one........... A photon interacts with a ground state electron in a hydrogen atom and is absorbed. The electron is ejected from the atom and exhibits a de Broglie wavelength of 5.908×10−10 m. Determine the frequency (in hz) of the interacting photon.
anyone want to help?
Answers
You need to be aware of a relationship between frequency and wavelength to answer this, but once you know that relationship the question is really straightforward, there isn't any calculating involved as it is a simple conversion from one to the other. This relationship is inversely proportional. Mathematically this is can be wrote as 1/x = y, in this question we can write this as wavelength = 1/frequency or frequency = 1/wavelength, plug in the value of the wavelength 5.908x10^-10 m and you can find the value of the frequency. Remember, you don't need an equation to solve this type of question, just remember that relationship. Hope this helps.
27 January 2013
What happens to electron depends on energy of photon. It may be ejected from atom or may go in higher or excited state. this creates ionized atom and electron or an excited atom.
e
12 February 2021
Daniel Deck's answer may be incorrect. It seems that he is describing the relationship between wavelength and wave number, which is indeed reciprocal. The relationship between wavelength and frequency (i.e. distance and time), involves the velocity, as would be expected from the units. That relationship is described by the equation:
velocity (ms^-1)
= wavelength (m) x frequency (s^-1)
Since we are dealing with em radiation, velocity is the speed of light, c (3 x 10^8 ms^-1). So, rearranging the equation and substituting In c and the de Broglie wavelength, we have:
frequency = 3 x 10^8 / 5.908x10^-10
= 5.078 x 10^17 Hz.
28 November 2023
Alage produces ATP = Photon - Photon Exchange?
08 June 2025

Add an answer
Similar questions
 .
. 

 reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate
reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate  is generated. The equation of this reaction is given below:
is generated. The equation of this reaction is given below: