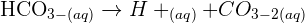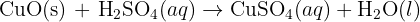Can anyone help
I have a diff problem .......Titanium (Ti) can be produced by the reaction of metallic sodium (Na) with titanium tetrachloride vapor (TiCl4). The byproduct of this reaction is sodium chloride (NaCl). Calculate the amount of titanium produced (in kg) when a reactor is charged with 73.0 kg of TiCl4 and 10.0 kg of Na.
Answers
The Molar Mass of is and the Molar Mass of is
So:
Divided by a to turn grams into kilograms
We end up with:
of formed
21 November 2012

Add an answer
Similar questions
 .
. 

 reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate
reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate  is generated. The equation of this reaction is given below:
is generated. The equation of this reaction is given below: