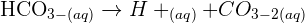Answers
Boiling is when a compound turns from a liquid into a gas. As polystyrene is a polymer it contains very large molecules which would require a lot of energy to separate and turn into a gas so the boiling point would theoretically be incredibly high. But at this high temperature the chains themselves would have broken down so polystyrene doesn't have a boiling point only a melting point.
02 December 2016
One reason that you might not find a boiling point is that the styrofoam might break down ( decompose ) so that bonds break WITHIN the chain of the molecules before all the forces between the chains break ( so that it would boil ).Another reason is that the chains are all different lengths and so different masses. This may mean that the styrofoam may melt over a range of temperatures.
02 January 2017
240 C
06 January 2017
430°C
25 April 2021

Add an answer
Similar questions

 .
. 

 reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate
reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate  is generated. The equation of this reaction is given below:
is generated. The equation of this reaction is given below: