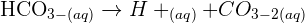Understanding the Reaction between Copper Oxide and Sulphuric Acid
The reaction between copper(II) oxide and sulphuric acid is a classic chemistry experiment often used to demonstrate how acids react with metal oxides.
In this reaction, a base (the metal oxide) reacts with an acid to produce a salt and water. This specific reaction is widely used in schools to prepare crystals of copper(II) sulphate.
Reactants and Products
To understand this reaction, we must first identify the physical states and colours of the chemicals involved:
- Copper(II) oxide (CuO): This is the reactant. It is a black solid powder. It is a base because it can neutralise an acid.
- Sulphuric acid (H₂SO₄): This is the acid. It is typically used as a clear, colourless aqueous solution (dissolved in water).
- Copper(II) sulphate (CuSO₄): This is the product (the salt). In solution, it is a characteristic bright blue colour.
- Water (H₂O): This is the liquid by-product.

Chemical Equation
We can write this reaction in two ways.
Word Equation: Copper(II) oxide + Sulphuric acid → Copper(II) sulphate + Water
Balanced Symbol Equation: The symbol equation including state symbols is:
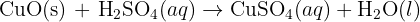
What Type of Reaction Is It?
This reaction is classified as a neutralisation reaction.
Neutralisation occurs when an acid reacts with a base. In this case, the copper(II) oxide acts as the base (specifically a metal oxide) and the sulphuric acid provides the hydrogen ions (H⁺).
The general formula for this type of neutralisation is:
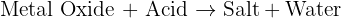
Practical Overview & Observations
If you were to carry out this reaction in a laboratory to create copper sulphate crystals, you would follow these steps:
- Heat the acid: Warm dilute sulphuric acid in a beaker (do not boil it).
- Add the base: Add the black copper(II) oxide powder to the acid a little at a time, stirring constantly.
- Observe: The mixture will turn from colourless to blue.
- Excess: Continue adding copper oxide until no more reacts. You will see black powder sitting at the bottom of the beaker. This ensures all the acid has been neutralised.
- Filter: Filter the mixture to remove the excess black copper oxide. You are left with a clear blue solution of copper(II) sulphate.
The most important visual change is the black solid disappearing and the solution turning a transparent blue.
Wear safety goggles to protect eyes from the acid.
Handle warm acid with care to avoid burns.
Summary
- Reactants: Black copper(II) oxide solid and colourless sulphuric acid.
- Products: Blue copper(II) sulphate solution and water.
- Type: Neutralisation (Acid + Metal Oxide).
- Observation: Black powder dissolves; solution turns blue.

 .
. 

 reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate
reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate  is generated. The equation of this reaction is given below:
is generated. The equation of this reaction is given below: