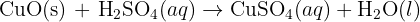How Does Carbon Dioxide React with Lime water?
You may often come across a question "What gas turns limewater cloudy?" The answer to this question is well known. Carbon dioxide is the only gas that turns lime water cloudy. You may be wondering what is lime water used for. Limewater is an aqueous solution of slaked lime and you will find it in antacids, medicines and lotions. But one of its most noteworthy property is that it is used to absorb carbon dioxide from the air. In this article, we have answered all the questions related to the reaction of lime water and  .
.
What happens when Lime Water reacts to Carbon Dioxide?
Carbon dioxide reacts with limewater to form calcium carbonate, which precipitates out of the solution. The carbon dioxide and limewater react to produce water in addition to the calcium carbonate. Calcium carbonate is chalk, and when it is produced, it precipitates and solid particles of chalk appear. The appearance of this solid makes the liquid appear ‘milky’. The white milky suspension/precipitate is caused by the formation of calcium carbonate. The characteristic carbon dioxide test, is checking that the limewater is milky. This is because chalk is precipitating in the limewater. Bubbling carbon dioxide through the solution for an extended period of time makes the solution become clear and colorless. This happens as the carbon dioxide forms acidic carbonic acid when it dissolves in the water, the carbonic acid (H2CO3) reacts further with the calcium carbonate. This chemistry is important in understanding how hard water is formed and then limescale is formed in kettles and hot water boilers. 
Written as an Equation
In its equation form that makes:

How to test for carbon dioxide?
Now, we will answer how to test for carbon-dioxide. One of the most effective ways to test for carbon dioxide gas is the limewater test. When carbon dioxide reacts with lime water (calcium hydroxide solution), a white precipitate of calcium carbonate is produced. The solution of calcium hydroxide is limewater and if carbon dioxide bubbles through the limewater, it turns cloudy white or milky.
How does carbon dioxide turns lime water milky?
 reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate
reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate  is generated. The equation of this reaction is given below:
is generated. The equation of this reaction is given below: 
Now, the question arises why the solution turns milky. Well, the answer is simple, The reason for the milky solution is that calcium carbonate which is produced as a result of this reaction is a white precipitate. This nature of calcium carbonate also helps us to test for the presence of carbon dioxide gas. All you have to do is to bubble the gas through a solution of calcium hydroxide. If the gas is carbon dioxide, then the solution will turn milky. If not then the gas which is subjected to the test is not carbon dioxide. If you continue to bubble the carbon dioxide gas through limewater, you will witness another acid-base reaction that will dissolve the precipitate to generate soluble calcium hydrogen carbonate. The equation of this reaction is given below:

What is the reaction between carbon dioxide and water?
When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. Limewater and  reaction results in a carbonic acid. It is a weak acid and it is in an aqueous form, i.e., it is a water solution. The chemical equation of this reaction is given below:
reaction results in a carbonic acid. It is a weak acid and it is in an aqueous form, i.e., it is a water solution. The chemical equation of this reaction is given below:

Since it is a weak acid, therefore some of it dissociates to generate H+ ions. This depicts it is a slightly acidic solution that forms hydro carbonate ion.

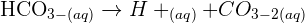
All of these reactions are reversible.
Does lime water absorb carbon dioxide?
Yes, limewater absorbs carbon dioxide. When lime water and carbon dioxide reacts, calcium carbonate is generated along with the water. Calcium carbonate is an insoluble salt. The equation of this reaction is given below:

Why is lime water used in experiments?
Limewater is used in experiments because it is the easiest way to detect the presence of  gas. Limewater is a calcium hydroxide solution that produces a white precipitate of calcium carbonate when it reacts with carbon dioxide. The white precipitate can be easily detected by the person conducting the experiment. Due to this fact, you will often see that limewater is used to detect the presence of carbon dioxide.
gas. Limewater is a calcium hydroxide solution that produces a white precipitate of calcium carbonate when it reacts with carbon dioxide. The white precipitate can be easily detected by the person conducting the experiment. Due to this fact, you will often see that limewater is used to detect the presence of carbon dioxide.