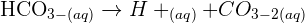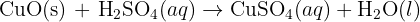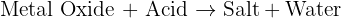show that calcium carbonate is stable at room temperature?what does this mean and how do i show it
show that calcium carbonate is stable at room temperature?what does this mean and how do i show it
Answers
All the metal carbonates in Group 2 undergo thermal decomposition to give the metal oxide and carbon dioxide gas. As you go down Group 2, the carbonates have to be heated more strongly before they will decompose. So calcium carbonate is more stable than magnesium carbonate and would need to be heated more strongly in a test tube than magnesium carbonate before it decomposes .
20 May 2013

Add an answer
Similar questions
 .
. 

 reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate
reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate  is generated. The equation of this reaction is given below:
is generated. The equation of this reaction is given below: