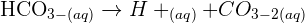Gas phase reaction help
The gas-phase reaction between trichloromethane, CHCl3, and chlorine, Cl2, has time-independent stoichiometry and can be represented as follows:
Equation 1 CHCl3(g) + Cl2(g) = CCl4(g) + HCl(g)
Under certain experimental conditions, the experimental rate equation was found to be:
Equation 2 J = kR[CHCl3][Cl2]^1/2
A three-step mechanism has been proposed for Reaction 1:
Equation 3 k1 Cl2 ↔ 2Cl• k-1
Equation 4 k2 CHCl3 + Cl• → HCl + CCl3•
Equation 5 k3 CCl3 + Cl• → CCl4
(i) Explain why the form of the experimental rate equation indicates that Reaction 1 cannot be an elementary reaction.
(ii) With reference to the three-step mechanism (Equations 3–5 ), and assuming that the second step (Equation 4) is rate-limiting, derive the chemical rate equation for this mechanism and then compare it with the experimental rate equation given in Equation 2.
(iii)The activation energy for the forward reaction, Ef , of step 1 (Equation 3) is 243.4 kJ mol^-1. Given this information, determine the activation energy for the reverse reaction, Er, and comment on the significance of the value (one sentence only).

 .
. 

 reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate
reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate  is generated. The equation of this reaction is given below:
is generated. The equation of this reaction is given below: