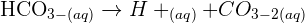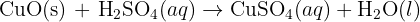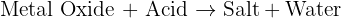Answers
This is a neutralization reaction that forms CuSO4. CuSO4 is sky blue.
17 May 2019
U will observe a blue precipitate plus a gas co2 that will turn lime.water milky.
18 May 2019
The colour change will be from colourless to blue. CO2 is formed which if put in lumewater will turn the colour of the solution milky white which is an indication of the formation of the reaction.
29 May 2019
Blue
28 June 2019
Blue
28 June 2019
Copper carbonate reacts with Sulphuric acid (reactants) giving rise to Copper sulfate (CuSO4) (residue, powder), Carbon dioxide (CO2)(gas) and Water (H2O)(liquid). This is a neutralization reaction, COPPER SULFATE IS BLUE IN COLOUR.
05 July 2019
the white CuCO3 powder when it reacts with H2SO4 will turn blue
08 July 2019
CuCO3 is white coloured. When it reacts with sulfuric acid, the white solution changes into blue. This is due to the formation of Copper sulphate.
27 July 2019
the color change during this reaxction is black to white. as we know the color of copper carbonate is black and when we add sulphuric acid to it. the reaction procced to form anhydrous copper sulphate. which is white in color
08 August 2019
When H2SO4 is added to CuO, the CuSO4 that forms dissolves in water. The color of the mixture changes from______ to _______.
01 December 2020
Someone should help please, when CO2 to bubble H2SO4 what will be the reaction
20 August 2021
from black to blue
09 June 2024

Add an answer
Similar questions

 .
. 

 reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate
reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate  is generated. The equation of this reaction is given below:
is generated. The equation of this reaction is given below: