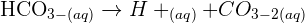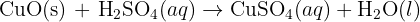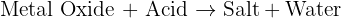why is calcium oxide more hazardous than calcium hydroxide?
why is calcium oxide more hazardous than calcium hydroxide?
Answers
Calcium Oxide is a solid with a very high affinity for water - it will react with water in the air, or in your skin or anywhere it can and form calcium hydroxide. This reaction is exothermic so it releases a lot of heat while it is reacting - there fore as well as being corrosive and causing significant skin irritation, calcium oxide's reaction with water can also cause burns. Calcium hydroxide is basically hydrated calcium oxide. It is alkali so can be corrosive. In solution it makes limewater. Does that help?
19 March 2017

Add an answer
Similar questions
 .
. 

 reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate
reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate  is generated. The equation of this reaction is given below:
is generated. The equation of this reaction is given below: