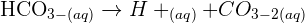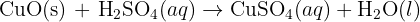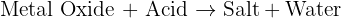Why are d-block elements not as reactive as s-block elements
Why are d-block elements not as reactive as s-block elements
Answers
D block elements tend to have a more stable outer ring of electrons, when you reach D block elements they start adding electons to inner rings rather than outer so the added electrons create more stability. S block however are still lacking electrons so group 1 elements wish to lose their electrons as quickly as possbile making them very reactive, and group 7 wish to gain an electron.
07 August 2017
Atoms have shells of electrons, a bit like layers of an onion. Atoms are most stable when they have full shells. The shells fill from inner to outerwards. The order is: 1st shell: 2s2nd shell: 8 (2s + 6p)3rd shell: 18 (2s + 6p + 10d)4th shell: 32 (2s + 6p + 10d + 14f)The s shell is the closest to the atom, and holds upto 2 electrons. When an atom is an s block elements, the atom has a full shell along with 2 s electrons in the next and most outwards shell. The easiest thing to do, in order to achieve stability, is to lose the 2 electrons. This is done by joining with another atom and donated the 2 extra electrons. The smaller the number of extra electrons, the easier they are to lose, and the easier it is to then end up with a full outer shell. This is why the s-block elements are so reactive.
12 August 2017
The reactivity of elements reduces as you move from left to right in a periodic table. S-block elements have one or two electrons in the outer most shell (valence shell) which makes them easier to move/share while reacting with other elements. On the other hand, in D-block the valence shell electron increases and its gets litter bit harder for them to react as compare to S-block elements.
15 August 2017
The transition metals are characterized by partially filled d subshells in the free elements and cations. The ns and (n − 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. In the second- and third-row transition metals, such irregularities can be difficult to predict, particularly for the third row, which has 4f, 5d, and 6s orbitals that are very close in energy. The increase in atomic radius is greater between the 3d and 4d metals than between the 4d and 5d metals because of the lanthanide contraction. Ionization energies and electronegativities increase slowly across a row, as do densities and electrical and thermal conductivities, whereas enthalpies of hydration decrease. Anomalies can be explained by the increased stabilization of half-filled and filled subshells. Transition-metal cations are formed by the initial loss of ns electrons, and many metals can form cations in several oxidation states. Higher oxidation states become progressively less stable across a row and more stable down a column.The s-block elements are the 14 elements contained within these columns. All of the s-block elements are unified by the fact that their valence electrons (outermost electrons) are in an s orbital. The s orbital is spherical and can be occupied by a maximum of two electrons. Elements in column 1 have one electron in the s orbital, and elements in column 2 (plus helium) have two electrons in the s orbital.
17 August 2017
The transition metals are less reactive than s block elements. This is due to their higher heats of sublimatiin , higher ionization energies and lesser hydration energies of their ions.
09 November 2020
Which is the most reactive agent in d block of modern periodic table?
07 June 2022

Add an answer
Similar questions
 .
. 

 reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate
reacts with the carbon dioxide gas, an insoluble solid known as calcium carbonate  is generated. The equation of this reaction is given below:
is generated. The equation of this reaction is given below: