Chapters
Atoms are building blocks of all non-living things and substances. Every matter around us consists of atoms. They are so small that they can't even be viewed through a simple microscope. Special microscopes are made that magnify things up to millions of times. The question is, what is an atom made up of?

What are Atoms Made Up Off?
If you look at an atom, you will see a prominent ball-type structure; small balls are rotating around that structure. The big ball is called the nucleus, and the small ball is called the electrons.
An atom is comprised of three subatomic particles: proton, electron, and neutron. These three are the fundamental particles that make up an atom. In all atoms, you will find protons, electrons, and neutrons. Hydrogen is an exception because it doesn't have any neutrons.
The nucleus is made up of protons and neutrons. However, electrons revolve around the nucleus like Earth revolves around the sun. Let's understand the subatomic particles in detail.
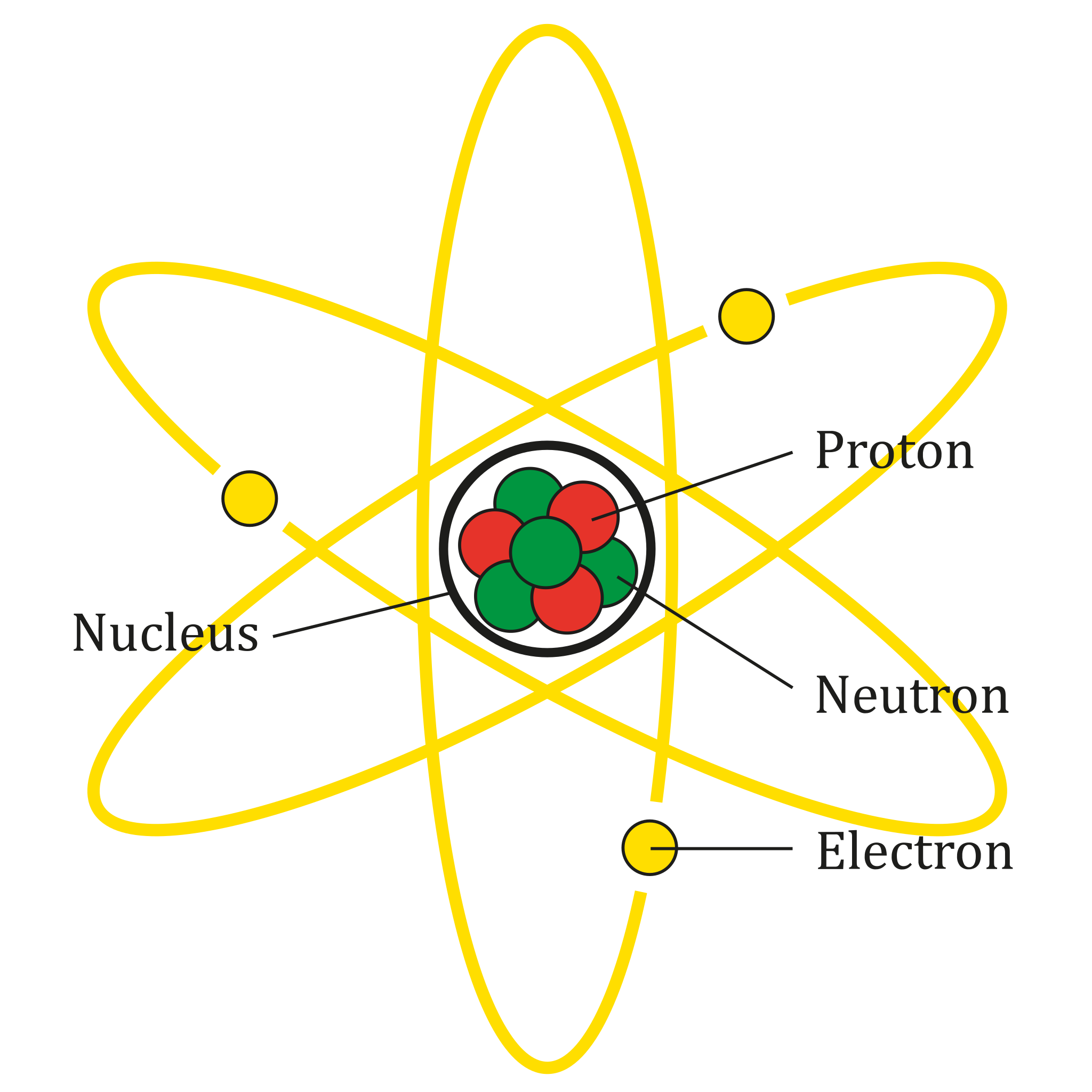
Protons
Protons are positively charged subatomic particles. They are found in an atom's nucleus of an atom, packed tightly with neutrons. Since protons are positively charged particles, they have a relative charge of +1. Since atoms are made up of matter and matter has weight, atoms also have weight. This means that subatomic particles will also have weight, and proton is no exception. The relative mass of a proton is 1. Proton plays a vital role in an atom. A change in the number of protons in the nucleus changes the whole atom. This means that the atom will exhibit different properties with just one proton change. Protons are fixed subatomic particles. Last, they are usually denoted by the letter "p".
Neutrons
Neutrons are another type of fundamental particle that is found inside an atom. Neutrons are neutral particles. It means a neutron has no charge and doesn't exert force like protons and electrons. Their relative mass is equal to 1. Neutrons are found in the nucleus with the proton. The whole weight of an atom is dependent on the protons and neutrons. Usually, neutrons are more than protons; therefore, most of an atom's weight is dependent on the neutrons. Neutrons' role is to tightly pack with the proton and increase the atom's weight. The symbol n denotes neutrons.
Electrons
An electron is the last fundamental particle in an atom yet to be discussed. Electrons aren't found in the nucleus; electrons revolve around the nucleus like Earth and other planets revolve around the sun. The accommodation of electrons is something that you will feel differently. An atom has a nucleus and shells. These shells are invisible circular paths where electrons move around the nucleus. Only electrons can move on these shells. The relative charge on an electron is -1. This charge balances out the proton's charge, making an atom neutral; however, atoms are stable if their outermost shell is filled. Therefore, electrons can jump/escape from the shell or accept a foreign electron unless the atom is stable. Hence, atoms share this electron transfer to keep themselves stable.
You might wonder if adding or losing electrons affects the atom's weight. The answer is no because the mass of an electron is almost equivalent to zero. The relative mass of an electron is equal to $frac { 1 }{ 1840 }$, which is very low. Think about it: you need 1840 electrons to increase the mass by one amu, which is a lot, and such an atom doesn't even exist. Last but not least, the symbol of an electron is e.
How To Understand Atoms?
Suppose you are provided with an atom, and this is what you see:
7Xa4
The above atom is a random atom we made. How do you learn about the number of protons, neutrons, and electrons just by looking at the symbol? It's pretty straightforward; this is how you can read an atom:
Nucleon Number (mass number)XaProton Number (atomic number)
The superscript of an atom is the nucleon number. Nucleon number is the sum of proton numbers and neutron numbers. Since both of them make up the mass of an atom, it is written as a sum, and a new name is given to it, and the name is nucleon number, also known as mass number.
Nucleon number (A) = number of protons (p) + number of neutrons (n)
In an atom, the number of protons always equals the number of electrons that balances out the force of attraction. Atoms do eject or accept electrons, but they will always have a charge on them. If there is no charge on the atom, then the number of protons will always equal the number of electrons. Let's get back to our atom example. We have an atom 7Xa4; the nucleon number is seven, and the proton number is 4. We still need to learn the number of neutrons and the number of electrons. As mentioned above, the number of electrons will equal the number of protons if the atom doesn't have a charge. Our atom has no charge; hence, the number of electrons will be 4. The last one remaining is the number of neutrons, which we can easily find from the number of nucleons.
Nucleon number (A) = number of protons (p) + number of neutrons (n)
7 = 4 + n
7 - 4 = n
n = 3
Hence, the number of neutrons is 3. This is how you find the subatomic particles. Below is the table for all subatomic particles with their symbols, relative mass, and charge.
| Particle | Symbol | Relative Mass | Charge |
|---|---|---|---|
| Proton | p+ | 1 | +1 |
| Neutron | n0 | 1 | 0 |
| Electron | e- | 1/1840 | -1 |
Isotopes
For example, you have two atoms. Atom "X" has a nucleon number of 8 and a proton number of 5. Meanwhile, the atom "Y" has a nucleon number of 10 and a proton number of 5.
8X5 & 10Y5
Did you notice something? Both atoms have the same proton numbers. We mentioned above that the number of protons is the unique information of an atom; with a change in the number of protons, the whole atom changes. Neither electron nor neutron changes an atom on their increase or decrease, but the number of protons does. But then, the above atoms have the same proton number, which means both are the same. Is this suitable? However, the number of nucleons is different, meaning the number of neutrons is not the same for both atoms. How is this possible? Same atoms but with a different number of neutrons? Yes, it is possible, and we call such atoms isotopes. Isotopes are atoms with the same element, with the same number of protons but different neutrons.
Isotopes are the same atoms, but the fact that both atoms have different numbers of neutrons brings some changes in the atom. For example, we have two isotopes of hydrogen.
1H1 & 2H1 & 3H1
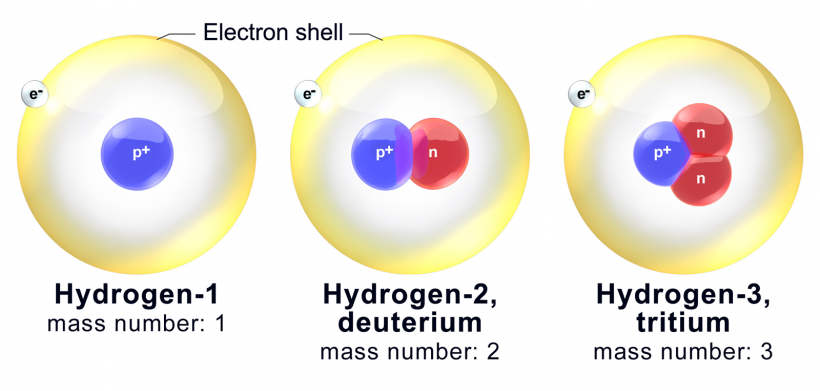
1H1 is the ordinary hydrogen you can find anywhere, with 1 proton, 1 electron, and zero neutrons. The second isotope is the deuterium, 2H1, which contains 1 proton, 1 electron, and one neutron. The third isotope is the tritium, 3H1, which contains 1 proton, 1 electron, and two neutrons. The number of protons remained the same, but the number of neutrons was changed in all isotopes.
Properties of Isotopes
Isotopes are the same element, having the same number of protons and electrons but different numbers of neutrons. The increase or decrease in neutron number changes the element's physical properties. The chemical reaction only involves electrons, and isotopes have the same number of electrons. Therefore, isotopes have similar chemical properties. For example, chlorine-35 and chlorine-37 are both isotopes, but the reaction with sodium to produce sodium hydroxide will be the same for both isotopes.
However, physical properties will change because of the mass. Isotopes involve changes in mass that result in different physical properties. For example, deuterium2H1 contains an extra neutron that gives deuterium a slightly higher point and density than hydrogen.
Uses of Isotopes
Earth has many isotopes of different elements but in different compositions. Some isotopes are abundant, while some are rare. However, these isotopes are used in various fields of applications. Below are some examples of isotopes that are used in different fields.
- Technetium-99 is used to detect tumours.
- Iodine-131 is used in the treatment of thyroid disorder
- Californium-252 is used to detect explosives.
- Americium-241 is used in smoke detectors.
- Carbon-14 is used to estimate the age of things that contain carbon.
- Uranium-23 is used to estimate the age of rocks.
The above list is just a small list of how isotopes are used in various fields. There are so many isotopes that are used for different purposes. However, isotopes are usually radioisotopes. That means that they are radioactive substances. This means they emit radioactive waves that are dangerous to health. This radiation causes damage to living cells that results in cancer. That is why it is better to be safe and approach with safety if working with isotopes.
Summarise with AI:













Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic