In the previous resource, you learned how to make atomic structures and electronic configurations, but mastering these requires practice. The more you make atomic structures and electronic configurations, the more you master them. That is why we thought to give you some tasks relating to atomic structures and electronic configuration in this resource. In this resource, you will practice making atomic structures and electronic configurations.

Atomic Structures
In the last lecture, you learned about protons, neutrons, and electrons and how they work together in an atom. You also learned about their visual representation. It is time to move forward to practice it, in short, to master it. Our objective is to teach you to a limit so that if you are provided with any element, you can quickly draw its structure.
Let's start with ease. You are given hydrogen. The first thing you need to do is to check the element on the periodic table. Once you find it, copy the whole information and write it down separately for your ease.
1H1
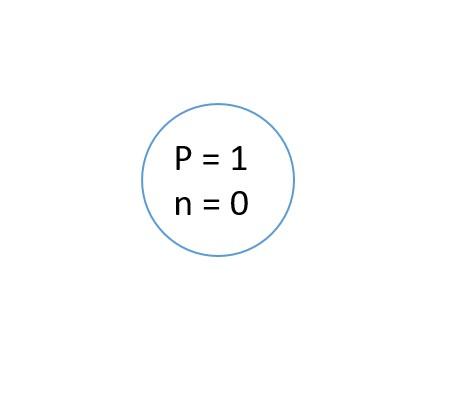
Extract the vital information from it. Hydrogen has one proton and one electron and contains zero neutrons. It means the nucleus contains only one proton. Make a small circle representing a nucleus and write down p and n. The small p stands for protons, and n stands for neutrons. Since hydrogen has no neutrons, n will be equal to zero, and p will be equal to one.
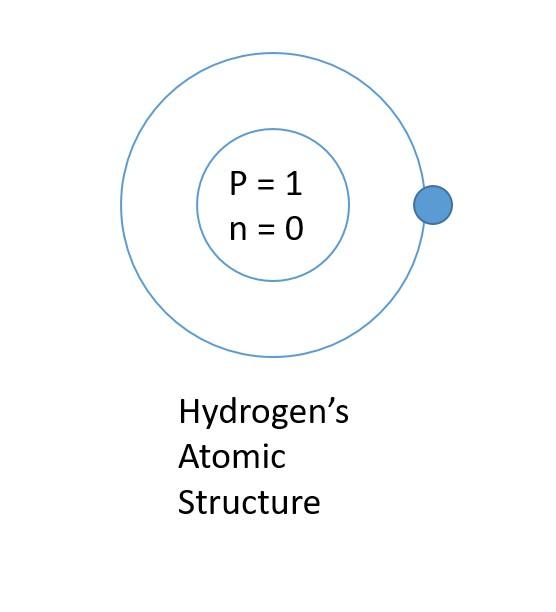
Once your nucleus is made, start drawing circles around it. Those circles are the shells of hydrogen. Draw two circles around the nucleus. Hydrogen contains only one electron; therefore, one electron is added to the shell closest to the nucleus, which is the first shell. Remember, the first shell will only contain 2 electrons and the remaining shell can be filled up to 8.
Now, why don't you give yourself a try? We have an element for you, and it is nitrogen. Why don't you try it, and then you can tally your answer with ours? Below is the structure of nitrogen.
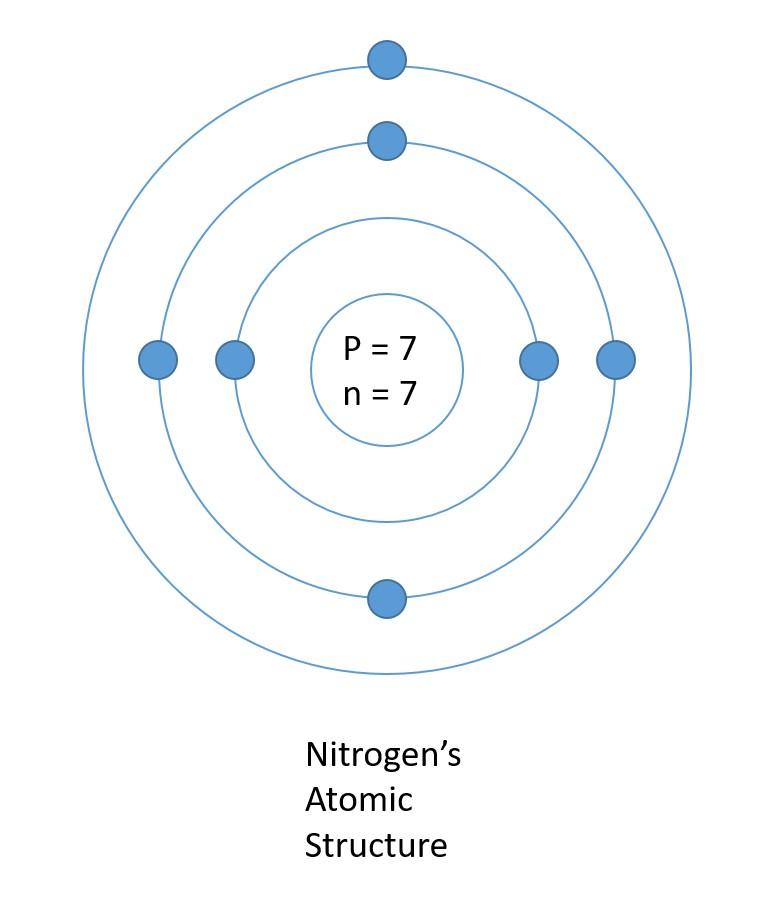
If you got it correctly, you can skip to the next paragraph; otherwise, here is the description of how we made the nitrogen element. According to the periodic table, the proton number is seven, and the mass is 14. That means 7 protons, seven neutrons (mass number- proton number) and 7 electrons. Make the nucleus and write down the proton and neutron numbers. The next step is to make the shells and accommodate the electrons. Make two shells; we will add more if necessary. Add two electrons to the first shell. We are left with 5 electrons now. Add those 5 electrons to the next shell.
Nitrogen atom was also pretty simple; let's move to a more complex element; how about sulfur? Give it a try, and then tally the answer. Below is the atomic structure of bromine.
If you made it correctly, then congratulations. You know how to make atomic structures, but if not, don't be hard on yourself; a time will come when you can draw it quickly. The key is to work hard and keep practising until you have mastered it; allow us to explain how to make sulfur structure. Find bromine on the periodic table and extract all the necessary information. Sulfur has 16 protons, and its mass is 32. That means 16 protons, 16 electrons, and 16 neutrons. Draw the nucleus of the sulfur and write the proton and neutron number. Draw the shells; since we are working with many electrons, drawing at least four shells is recommended. You will never be penalized by drawing extra shells. Draw as many as you want. Start filling up the shells with electrons. The first shell will have 2 electrons, leaving you with 14. Fill the next shell with 8 electrons. Still, you are left with 6 electrons. Fill the next shell with 6 electrons, and that is how you make the sulfur atomic structure.
Electronic Configuration
The electronic configuration will be straightforward if you learn to draw atomic structures. In the electronic configuration, you must show how electrons are arranged in their respective shells—for example, oxygen. The proton number of oxygen is 16; how do we know that? Because this was written on the periodic table. Always keep your periodic table when drawing atomic structures or making electronic configurations.
The first thing you need to do is check the element's proton number. For the case of oxygen, the proton number is 8. Remember, in elements, the number of protons will always equal the number of electrons. Therefore, the element oxygen has 8 electrons. Since you know the electrons, you can write the electronic configuration. Below is the electronic configuration of oxygen:
O = 2, 6
The first digit represents the number of electrons in the first shell. We all know that the first shell can only occupy 2 electrons; therefore, we wrote 2. The second digit represents the number of electrons in the 2nd shell. Except for the first shell, the remaining shells can accommodate 8 electrons. We have already placed 2 electrons in the first shell; the remaining electrons are six, and since the 2nd shell can place 8 electrons, fill it with the remaining electrons of oxygen, i.e. 6. The valance shell of this configuration is two because the valance shell means the last shell where at least one electron is occupied. In the above case, it is the second.
Let's do another example: you are asked to make the electronic configuration of magnesium. The proton number of magnesium is 12, meaning there are 12 electrons.
Mg = 2, 8, 2
The first shell will be filled with 2 electrons, and the remaining shells will be filled with remaining electrons. Only 10 electrons remained after filling the first shell; fill out the remaining shell. The valance shell of the above configuration is three because the third shell is the last shell that occupies the last set of electrons.
One last example for clarification: why don't you make the electronic configuration of calcium?
Calcium has 20 electrons. Fill up the shells in their respective manner.
Ca = 2, 8, 8, 2
The first shell requires 2 electrons. The remaining 8 electrons were required, and we filled it according to the number of electrons left. The valance shell is the 4th shell because the 4th shell is the last shell that occupies electrons.
Summarise with AI:












Thank you very much,you made me to understand in less than 20 minutes what I was unable to get during my secondary school days
Hi Dorcas! Thank you for your comment, great to hear that you found this resource helpful :)
Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic