Chapters
Ever wondered what things are made up of? Take an example of your smartphone. What is inside your smartphones that allows you to perform so many operations. Your smartphone consists of many electrical components and each component has its own and separate functionality. However, what is inside those electronics? That is a mystery? In fact, what is everything is made up of? Let's reveal this secret.

What is an Atom?
An atom is a small individual particle that can take part in a chemical reaction. Basically, every non-living thing is made up of atoms. Remember the particles that we talked about in states of matter? We were referring to atoms.
Just for the ease of understanding, imagine atoms as balls (although atoms are not balls, we will discuss it in the next heading). Each ball is attached together in a row and they are vibrating. Now imagine thousands of rows and columns. That is how matter is made up of. Atoms are very small that you can not even see it through a microscope. To see atoms, a special type of microscope is used which is only a few in this world.
If there are two or more than two atoms together then we will call it a molecule.
What Does an Atom Consists Off?
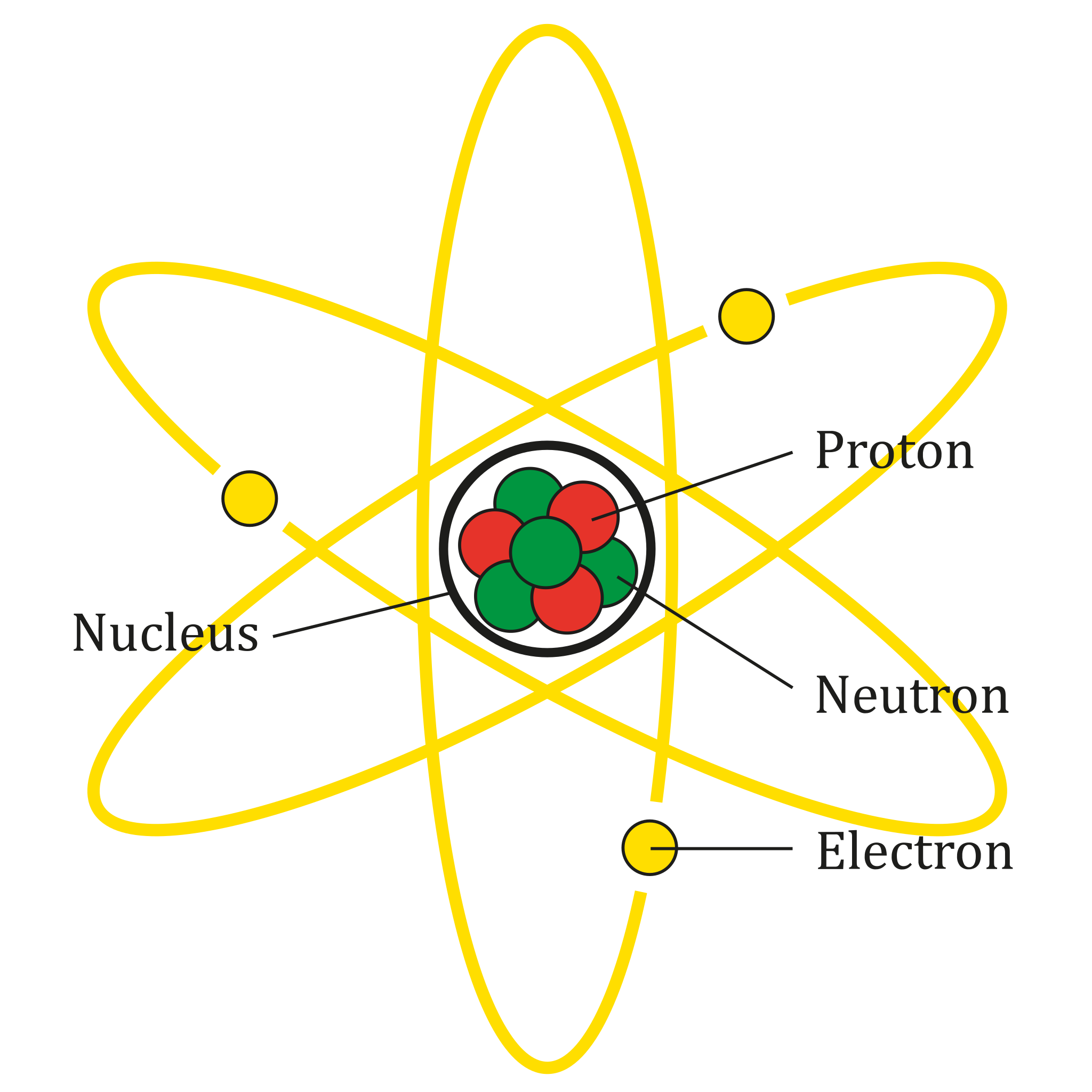
In the above paragraphs, we told you that atoms are balls but that is not the actual case. We made you visualize to teach you about atoms in an easy way. An atom consists of three subatomic particles and they are proton, neutron, and electron.
These three subatomic particles made up an atom, hydrogen is an exception. Protons are positively charged subatomic particles. Electrons are negatively charged subatomic particles and neutrons are neutral subatomic particles that contain no charge.
If you look at atoms, you will see two things. A big ball type object and small balls rotating around it. The big ball type object is called a nucleus. The nucleus consists of protons and neutrons and the small balls that are rotating around the nucleus are electrons.
Please note that hydrogen is the only element that contains no neutron. It contains only one proton and one electron.
| Particle | Symbol | Relative Mass | Charge |
|---|---|---|---|
| Proton | p+ | 1 | +1 |
| Neutron | n0 | 1 | 0 |
| Electron | e- | 1/1840 | -1 |
What is an Element?
At this point, you should have a clear idea about an atom otherwise you can't understand elements. The arrangement of subatomic particles in an atom is different from other atoms. For example, consider two atoms, atom A and atom B. Atom A contains 4 protons, 6 neutrons, and 4 electrons and atom B contains 3 protons, 5 neutrons, and 3 electrons.
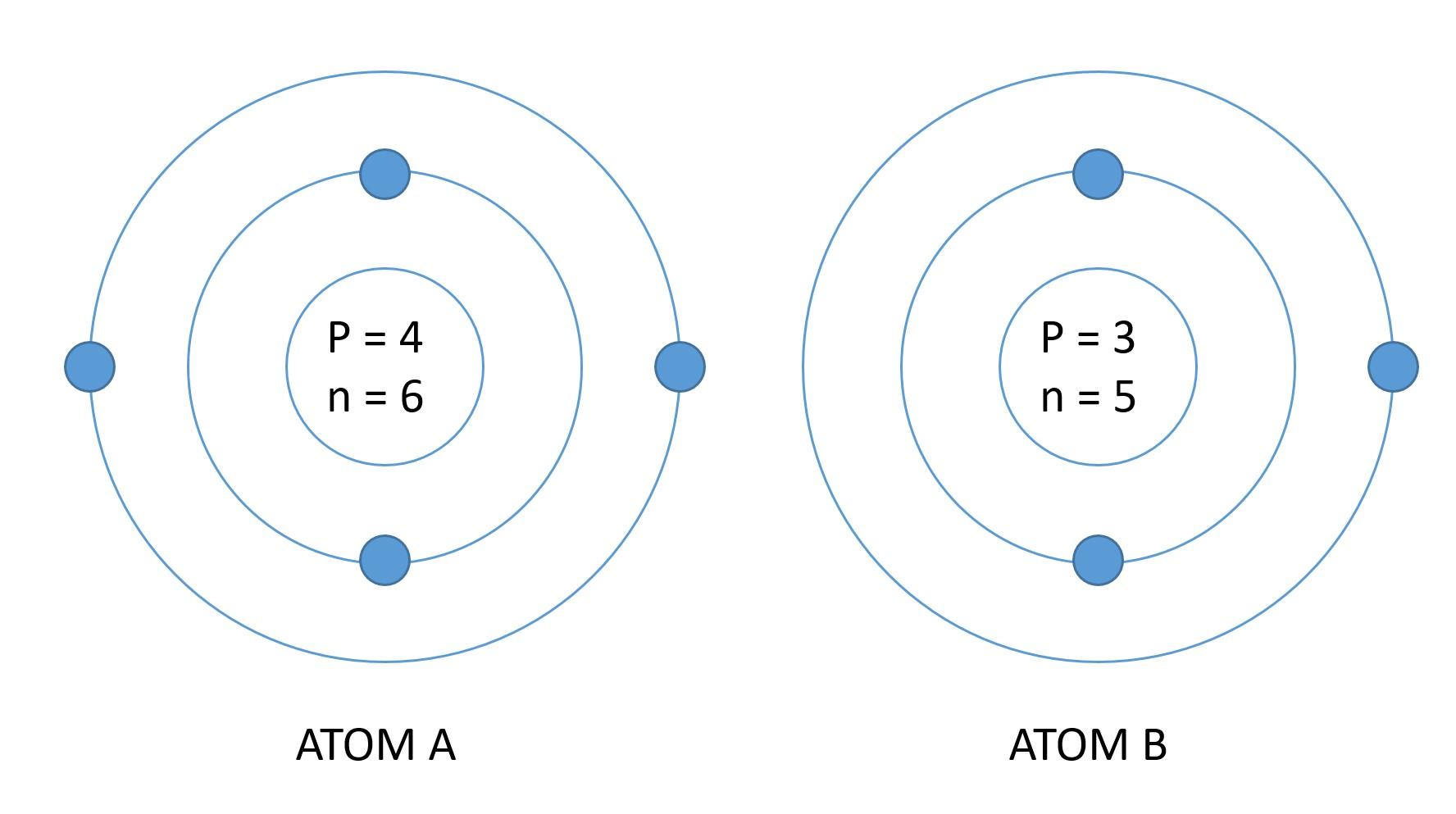
Number of protons, neutrons, and electrons are different in both atoms and because of this, their properties will also be different. That is why scientists categorized this arrangement and call them elements. The textbook definition of an element is that it is an atom that is indivisible and contains different sets of protons, neutrons, and electrons.
In an element, the number of protons will always be equal to the number of electrons to balance the force of attraction. Number of neutrons can vary from element to element. The most important thing that describes an element is the proton number. If you change the proton number, you change the whole element. For example, an element containing 2 protons is a gas, however, an element with a 3 proton number is solid. Do you see how a single proton changed the property?
Element categorization
There are 118 elements on this Earth. Each element is different from another and each element has its own name. Today, elements are recognized by their names. For example, the name of an element with one proton and one electron is hydrogen, the name of an element that contains two protons, two neutrons, and two electrons is helium and the list for 118 elements goes on. You can check the list of all 118 elements in the periodic table (which we will discuss in detail in the upcoming resource).
What is an Ion?
Let's say you have element lithium. Lithium has 3 protons, 4 neutrons, and 3 electrons. For some reason (which will be discussed in later resources), lithium ejected an electron and now lithium has 2 electrons. If we look at the overall force of attraction, there are more protons than electrons and that means that lithium will have a charge because of the unbalanced force of attraction. Since the proton charge is positive and lithium has more protons, therefore, lithium will have a positive charge. Lithium ejected one electron which means two electrons will neutralize the force of two protons but one proton remains idle therefore lithium will have one positive charge and it will be written as Li+.

The reason behind this whole example is to make things easier for you to learn. An ion is a charged particle. Lithium, which is charged positive, is an example of an ion. An ion is not an element, don't get confused. In an ion, number of protons will never be equal to the number of electrons.
In the above example, we showed that the number of protons was more than the number of electrons but this can be the opposite as well. Take an example of fluorine. Fluorine has 9 protons, 10 neutrons, and 9 electrons. This time, again for some reasons that will be discussed in upcoming resources, fluorine accepted an additional electron. Now fluorine has 9 protons and 10 electrons. The 9 protons will overcome the force of attraction of 10 electrons but one electron is remaining. Since the electron is a negative charged subatomic particle, hence the charge on lithium will also be negative. Only one electron was in excess that concludes lithium having -1 charge and it will be written as F-.
Types of Ions
There are two types of ions, positive ions and negative ions. Positive ions are those ions having a positive charge and negative ions are those ions having a negative charge. However, in chemistry, we use a special name for both ions and are cation and anion. Cations are positive ions and anions are negative ions.
Note: We know remembering both names with their identity isn't easy. That is why we have a trick up to our sleeves. Cations are positively charged ions and in short, we can write CP which is also an abbreviation of the class presentation. Therefore, remembering class presentations is easier. All you need is to remember the class presentation and then abbreviate it to CP and there you have it, cations are positively charged ions. Easy, right?
Difference Between Ions and Elements
Considering both are the same is one of the common misunderstandings. Ions and elements are two different things.
The most important point to note is that ions have charges while elements will never have any charge on them.
Furthermore, the number of protons will always be equal to the number of electrons in an element but in ions, number of protons will never be equal to the number of electrons.
Last but not least, elements are single neutral atoms while ion is charged particles.
Summarise with AI:












Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic