Chapters
Not every chemical is a compound. Some chemicals are different and this uniqueness makes them special. We call them mixtures. Let's understand what mixtures are and their properties.

What is a Mixture?
To understand mixtures we have a perfect example. Imagine a glass of water. You add salt to it and stirred. The salt is fully dissolved in water. The question is did the salt react with water forming a new product? The answer is no because the chemical formula of water remains the same just the taste is changed and that is not considered as a change in water. As a matter of fact, you can separate water and salt too. The purpose behind this example is to tell you that solution of salt and water is an example of a mixture.
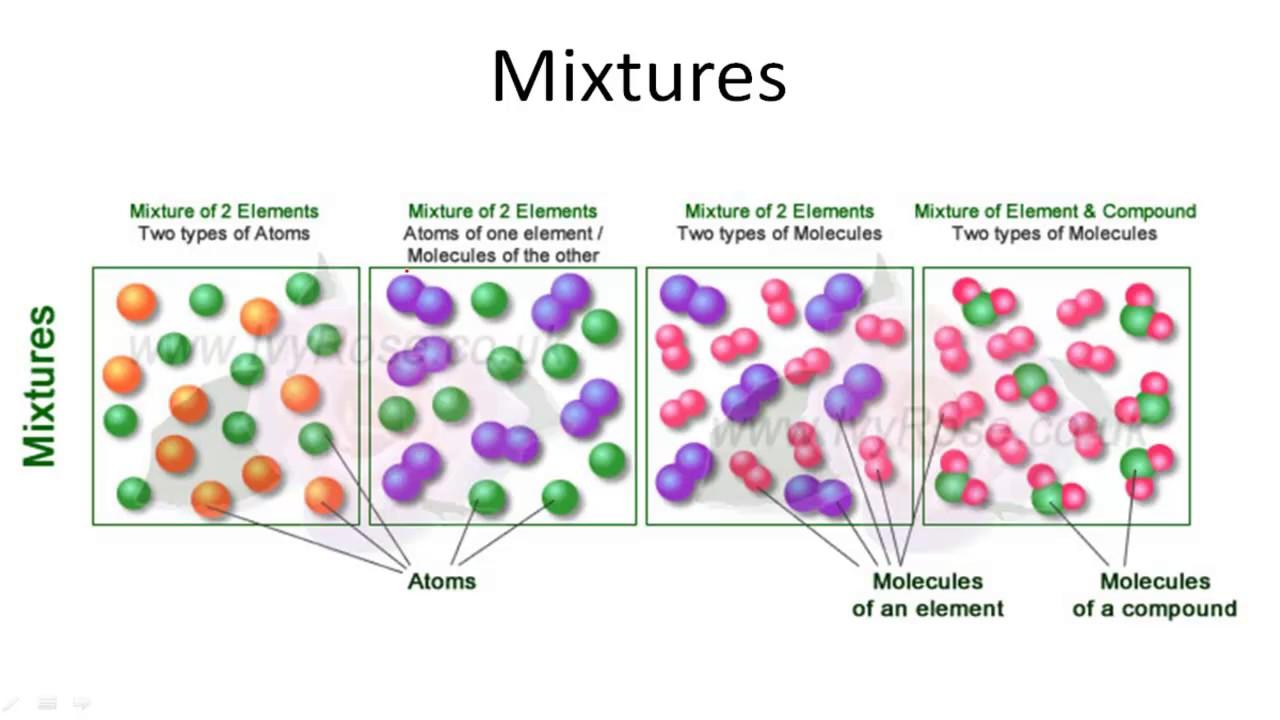
A mixture is the mixing of two or more elements or compounds that are not chemically combined.
Some of you might be wondering what is chemically combined? It means that the atoms of two or more substances make a bond with each other. This bonding is very strong and it also creates a new substance. To understand this more, please read our compound article.
In a mixture, there is no such thing as a chemical combination. It is just a physical combination. For example, a mixture of water and sand. Sand is insoluble in water. Did it bring any chemical change? Of course not, it is just a physical mixing.
How To Find Whether the Solution is a Mixture or Not?
This is a tricky question because one can not identify whether the sample is a mixture or compound by just looking at it. Sometimes it is very easy like sand and water, sometimes it is very hard like a solution of ethanol and water.
There is one way to identify whether the sample, that you are given, is a mixture or not. Mixtures can be separated. This involves different separation and purification methods (will discuss this topic in detail in upcoming resources). The method depends on the state of the sample. For example, to remove sand and water, you can apply the filtration technique and for the case of solution of ethanol and water, you can use the distillation method.
But one thing is for sure, mixtures will always get separated using different methods. If there is no separation, meaning you tried to separate the sample but you weren't able to separate then that sample is not a mixture.
Chemical Properties
When you mix two or more substances, their chemical properties will not change. Take an example of seawater. Seawater contains a high amount of salt. Does that change the formula of water? No, if you take a look at the molecular level, you will see water molecules and salt molecules moving separately in random directions. Hence, the chemical properties of a mixture will not change. It will remain the same.
Physical Properties
Since no reaction occurs therefore there will be no physical change in the physical properties. For example, when you add salt to the water, does the colour change? No, does water changes its state? Of course not. Water will remain water showing all those properties that water should. However, it is not the whole truth, in some cases, mixtures do affect physical properties. When salt was added to water, we didn't see any change except for one, the boiling point of the water increased. There are some exceptions that mixtures affect physical quantities but if you are asked whether mixture affects chemical or physical properties? Then you will always write no. The physical properties will remain the same.
Composition
Mixture is a physical mixing process of substances and this allows us to vary the composition of the substance according to your desire. For example, you got yourself a cup of coffee. You can adjust the sweetness by varying the amount of sugar. It depends on you how much you want to add. There is no limit in the composition.
Examples of Mixtures
Mixture is the only process where not much science is applied yet it is very important for you to understand about mixtures. In simple words, there are no rules for mixture except for one and that is chemicals should not undergo reaction. If they started reacting then the resulting product will not be a mixture.
This doesn't mean mixture has no meaning in this world. Without mixtures, many things would have never been invented. For example, alloys. Alloys are a mixture of metals with non-metals. Alloys have a wide range of applications such as aeroplanes, musical instruments, even it is used for medical and industrial purposes. Think about it, where would be us if we never discovered mixtures?
Some chemical mixtures examples are a mixture of iron and sulfur, ethanol dissolved in water, bromine diffused in the air, and many more. Some household examples of mixtures are salt and water, sugar and water, coffee, tea, carbon dioxide in carbonated drinks, and the list goes on.
Conclusion
Mixtures are made up of two or more two chemicals that are not chemically combined. The condition is no reaction should take place. Mixtures do not have properties of their own. It inherits properties from its substances. The properties include chemical and physical properties. Furthermore, the composition of the mixture is variable. It means that we can vary the amount of substances in the mixture according to our desires. Last but not least, to identify mixtures, check whether they can be separated or not. If the sample is separated into its components then we can declare the sample as a mixture.
We highly recommend you solve past papers after reading this article. This way you can evaluate how much you learned. Moreover, you will also understand the nature of questions that you can expect in your upcoming GCSE exams.
Summarise with AI:












Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic