At this point, you should know the differences between elements, compounds, and mixtures. If you have never learned about them or have weak concepts, then it is better to clear your concepts about them because we will use them to explain them.
Take the example of water. The chemical formula of water is H2O. You won't find this chemical formula on the periodic table because this isn't an element. You will find H and O in the periodic table but not H2O. This means that two elements have combined to form water. In chemistry, we say that hydrogen has made a bond with oxygen to form water. Let's understand the nature of bonding and the types of bonding that you will learn in detail in upcoming resources.

What is Bonding?
Let's take the example of two siblings. They share a bond; that is, they are united by blood. No matter how far they get, how badly they fight or how much they are separated, they will always have that bond. Just like this, chemicals also form a bond with other chemicals. Bonding is the force of attraction between two or more atoms to unite them. For example, just like you weld two metals to join them together, atoms also join together through bonding.
Reason Behind Bonding
At this moment, some of you might be wondering why chemicals make bonds. Why does hydrogen attach itself to oxygen? To understand this, you need to understand the nature of atoms.
Atoms always want to become stable and achieve stability. They need to fill their valance shell. For example, chlorine has seven electrons in its valance shell. It needs one more electron to achieve stability. Let's introduce another element called sodium. Sodium has one electron in its valance shell. It needs to donate one electron to achieve stability. In short, sodium needs to donate an electron, and chlorine needs to accept an electron. Like a jigsaw puzzle, both elements can work together to achieve stability. Therefore, sodium will donate its electron to chlorine, and chlorine will accept it. Sodium's electron is in chlorine. Therefore, they will make a bond.
In conclusion, bonds are created so that atoms can achieve stability. Achieving stability is the driving force behind bonding. Atoms don't mind attaching to other atoms until they get what they require.
How do you know If the chemical bonded or not?
This question is one of the most asked questions. Not every time atoms will join with themselves, such as oxygen making bonds with oxygen, etc. They might need to bond with different atoms with different chemical and physical properties. When different atoms (having different chemical and physical properties) combine, they will form a new compound. This compound will also have physical and chemical properties different from its parent atoms. This is not true for all cases, but this happens most of the time. For example, hydrogen and oxygen forms water. Hydrogen and oxygen are gases, while water is a liquid. This is just one difference; we can make a list of how water differs from hydrogen and oxygen.
The best way to find whether the product is a compound is to check whether it has chemical and physical properties different from its parent atoms. If you see changes in physical and chemical properties, your chemical is a compound. If it shows similar properties, then it is a mixture.
Types of Bondings
There are different types of bondings. Understanding each bond is essential, but in this section, we will only teach about those bondings included in the GCSE syllabus. According to the GCSE syllabus, you will learn these three types of bondings:
- Ionic bonding
- Covalent bonding
- Metallic bonding
The first two types of bonding are prevalent. You will learn about them in depth. However, this resource will give you a brief overview of all three types of bondings. Without any further ado, let's start, shall we?
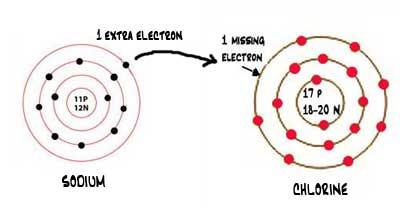
Ionic Bonding
Some of you might already guess what will happen in this bonding by reading the heading. Let's bring back our sodium and chlorine example. When sodium lost its valance electron, it got a positive charge, Na+, and when chlorine acquired that electron, it became an ion with a negative charge, Cl-. Due to both having opposite charges and not forgetting that sodium electrons revolve in the chlorine valance shell, they will attract each other, creating a bond. This bond will be called an ionic bond.
Bondings resulting from the transfer of electrons are called ionic bonds. The condition is that electrons should transfer like in sodium chloride. Ionic bonding occurs between metals and non-metals.
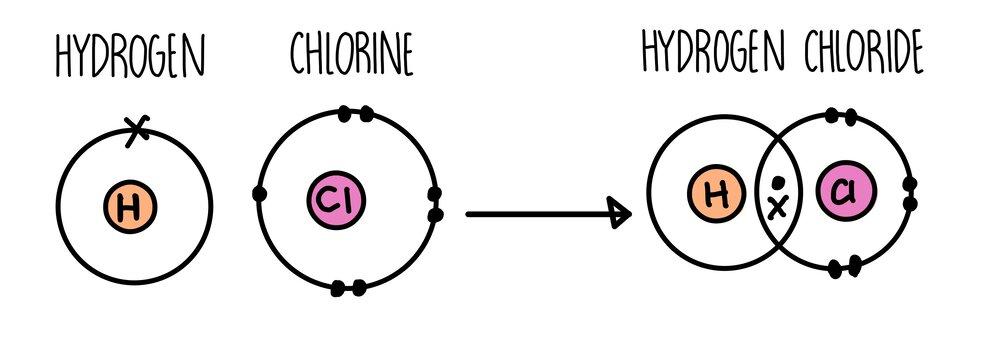
Covalent Bonding
Have you heard this phrase, "Sharing is caring"? This phrase is applied to covalent bonding. Not every time electrons are transferred, sometimes electrons are shared between two atoms. This way, both will become stable, and electrons will not need to be transferred. We call this sharing bonding covalent bonding.
To understand this bonding, take carbon dioxide as an example. Carbon is from group 4, having four electrons in the valance shell. Oxygen is from group 6, which means it has six electrons. Oxygen needs two more electrons to become stable; however, carbon needs four electrons to become stable. Therefore, they both agree that they will share a pair of electrons. This way, oxygen has two electrons, and carbon has two electrons rotating in both shells. However, carbon requires four electrons; two electrons are shared by oxygen, and carbon still needs two more. Therefore, carbon will make another agreement with another oxygen atom. This way, carbon gets stable, and so do both oxygen atoms.
If two or more elements or compounds share electrons/electrons, then bonds are formed, and this type of bonding is called covalent bonding. Covalent bonding occurs in non-metals.
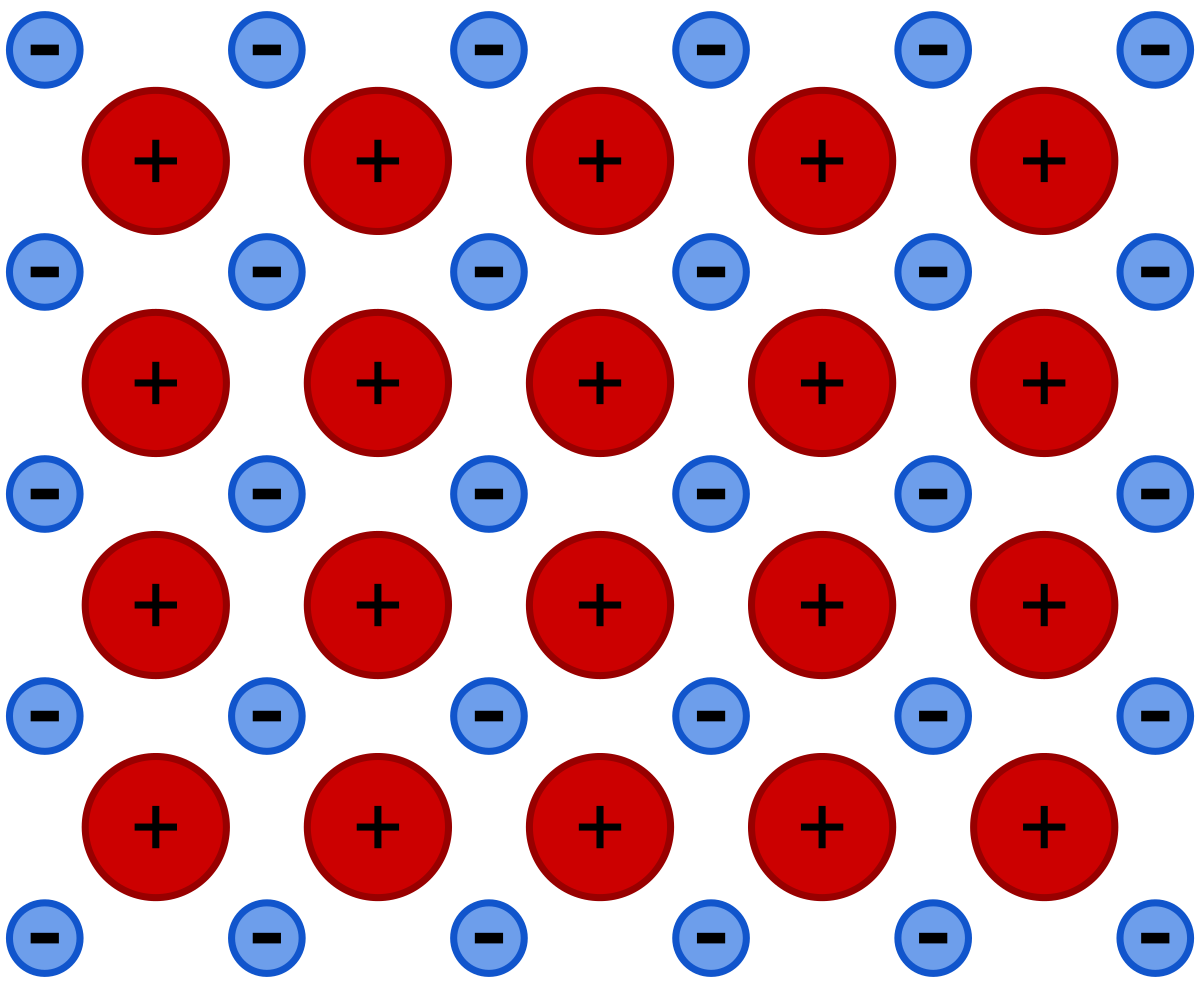
Metallic Bonding
This type of bonding occurs within metals. Metallic bonding is a force that holds atoms together. The atoms in the metallic bonding donate electrons and make themselves positive ions. This way, many atoms leave their electrons, making a sea of electrons. The force builds up between ions and valance electrons, resulting in the crystalline structure.
Summarise with AI:












Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic