Chapters
Metallic bonding is a fascinating concept that explains why metals behave the way they do. From the shimmering gold jewellery you wear to the aluminium foil in your kitchen, metallic bonding plays a crucial role. But what exactly is metallic bonding, and why is it so important? Let's take a look at this subject below. As we go along, you'll discover that metallic bonding is critical to unlocking many of the mysteries behind the everyday materials we often take for granted.

What is Metallic Bonding?
At its core, metallic bonding is the force that holds metal atoms together in a metallic substance. Imagine a sea of free-flowing electrons surrounding a structure of positively charged ions. These electrons are not tied to any particular atom; they can move freely throughout the metal. This unique arrangement gives metals characteristics such as electrical conductivity, malleability, and ductility. Think of it like a communal society where electrons are shared among a community of atoms, contributing to the greater good of the material's properties. However, it's important to mention that some metals don't always stick to the usual bonding rules. These exceptions make us question what we know and drive more research. The goal is to understand these unusual cases better, which could help scientists make discoveries in the science of metals.
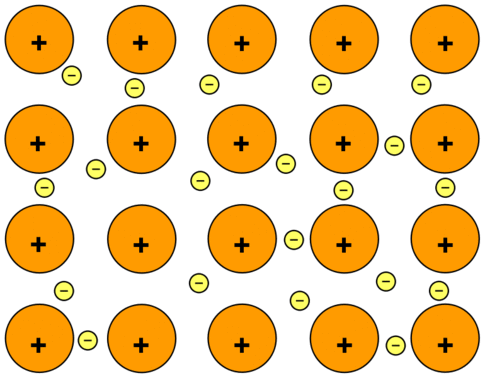
(Credit: Jim Clark, Libretexts)
The History and Evolution of the Concept
The idea of metallic bonding has evolved. Early theories suggested metals are like positive ions held together by a sea of negative electrons. However, these models were refined as our understanding of quantum mechanics grew. The "sea of electrons" model, introduced in the early 1900s by Paul Drüde, is still widely used today, although it's considered a bit simplified. In the modern era, scientists use advanced computational methods to study metallic bonding at the quantum level, providing insights unheard of just a few decades ago.
Metallic Bonding: Challenges and Controversies
The widely accepted "sea of electrons" model has critics who argue it's too simplistic for explaining complex quantum-level phenomena. For instance, the occurrence of superconductivity in certain metals at ultra-low temperatures challenges this traditional understanding. Additionally, the role of d-orbitals in transition metals like copper and gold is still a hot topic of debate. New materials such as metallic glasses and quasi-crystals also defy classical theories. These challenges aren't setbacks but opportunities for further research and are pushing scientists to refine existing theories and develop new models for a better understanding of metallic bonding.
The van Arkel-Ketelaar Triangle
The van Arkel-Ketelaar Triangle is a handy tool to understand bonding in a broader context. Firstly, it shows that chemical bonds are not just of a specific type but exist on a continuum. Metallic, ionic, and covalent bonds are the three extremes, and most compounds also exhibit varying degrees of these bonding characteristics.
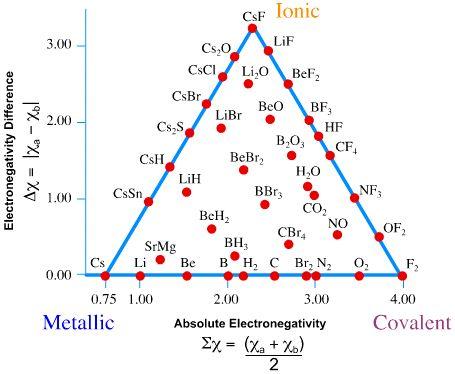
(Credit: Jim Clark, Libretexts)
Unique Properties of Metals
Due to the nature of metallic bonding, metals have several unique properties. Let's take a look at these below:
Electrical Conductivity: The free electrons allow metals to conduct electricity effectively. This is why metals are commonly used in electrical wiring and circuits.
Malleability and Ductility: Metals can be reshaped (malleable) and stretched (ductile) without breaking. This makes metals ideal for use in everything from car bodies to soda cans.
High Melting and Boiling Points: Strong metallic bonds require a lot of energy to break, resulting in high melting and boiling points. This is why metals are often used in high-stress environments like jet engines or industrial furnaces.
Luster and Color: Some metals, like gold and copper, have unique colours due to their interaction with light. This is another fascinating aspect of metallic bonding that contributes to the aesthetic value of these metals.
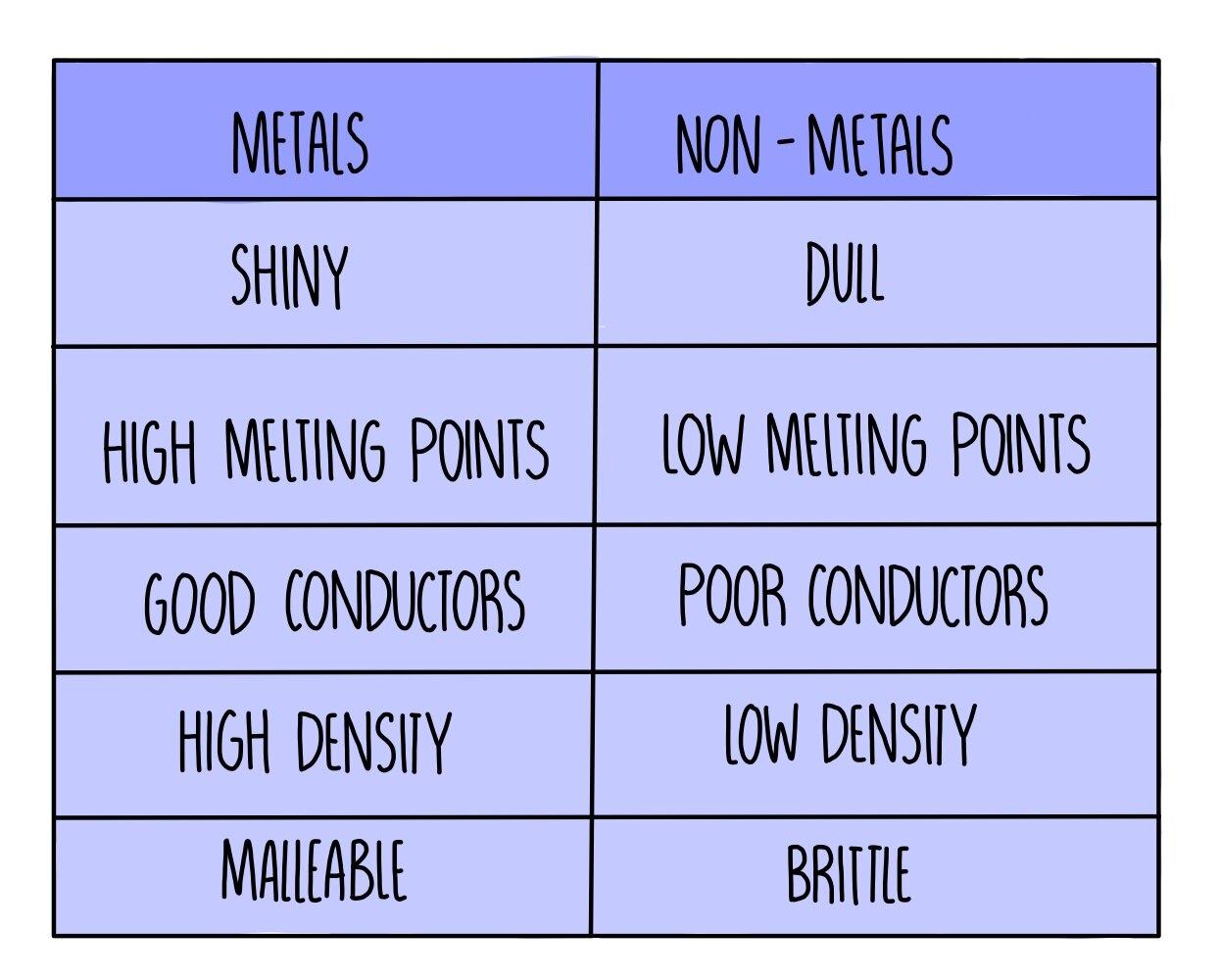
(Credit: thesciencehive)
The Role of Metallic Bonding in Alloys
One fascinating aspect of metallic bonding is in the creation of alloys. Alloys are mixtures of two or more elements, where at least one is a metal. The metallic bonds in alloys are similar to those in pure metals. Still, the presence of different elements can significantly alter the properties of an alloy compared to its component metals. For example, steel is an alloy of iron and carbon, where the presence of carbon atoms influences the metallic bonding between iron atoms. This results in a stronger and more durable material than pure iron. Understanding metallic bonding is crucial for materials scientists constantly developing new alloys for various applications, from construction to aerospace.
Real-world Applications
Metallic bonding has many real-world applications, too - it's crucial in various industries, from manufacturing to electronics and more. Understanding metallic bonding can also help study superconductivity, a phenomenon that could revolutionize energy transmission. Even in biochemistry, where weak interactions between metal ions and biomolecules can be significant, metallic bonding plays a role. For instance, the iron in our blood relies on metallic bonding principles to help transport oxygen throughout our bodies - isn't that incredible?
The Future of Metallic Bonding Research
As our technological capabilities continue to advance, the study of metallic bonding is beginning to enter new frontiers. What's more, With the help of nanotechnology, scientists can now tinker with metals at the level of individual atoms. This allows them to change the metal's bonding structure, offering new ways to improve its characteristics. In the future, this technology could pave the way for metals that are specifically designed to be stronger or lighter. Such advancements could revolutionize various fields, from creating fuel-efficient vehicles to constructing more durable buildings.
Conclusion
To sum it up, metallic bonding holds metal atoms together and is the reason behind their unique properties, like electrical conductivity and malleability. The "sea of electrons" model helps us understand this, but new research and technologies like nanotechnology are pushing the boundaries. By manipulating these bonds at the atomic level, we're opening the door to future metals with tailored characteristics. Alloys are a perfect example of how tweaking metallic bonds can create more robust or versatile materials. So, while we may think we know all about metals, studying metallic bonding shows there's still much more to discover!
Summarise with AI:












Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic