Chapters
Imagine a world without water, where diamonds lack their sparkle, and life as we know it ceases to exist. Sounds like a dystopian fantasy, right? However, this could be our reality if it weren't for the incredible power of covalent bonding. Intrigued? Discover how covalent bonding shapes our world and beyond - one atom at a time.

Why Covalent Bonding Matters in Chemistry
Covalent bonding is a central idea in chemistry, particularly important for non-metal elements commonly found on the right-hand side of the Periodic Table. When two atoms share electrons, they create stable molecules like hydrogen chloride. This process is essential for forming many biological compounds, including the proteins and nucleic acids that are the building blocks of life.
(Credit: BBC Bitesize)
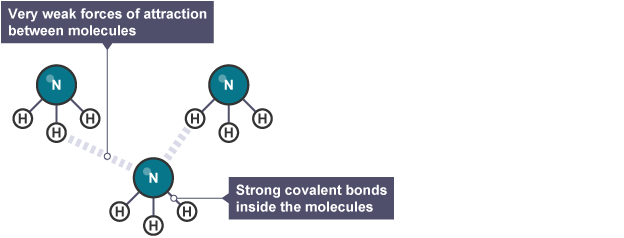
Small Yet Significant: The Role of Simple Covalent Molecules
Simple covalent molecules like water, carbon dioxide, and ammonia may be small, but their impact is massive! These molecules have unique properties that set them apart. For example, they often have lower melting and boiling points than ionic compounds. This is due to the weaker intermolecular forces acting between the covalent molecules. These simple molecules are also crucial for various chemical reactions that sustain life and power industries.
The Many Forms of Carbon
Carbon is a versatile element that forms a variety of covalent structures. Carbon's ability to form multiple types of covalent bonds makes it unique, from the hardness of diamonds to the flexibility of organic compounds. This versatility is crucial in fields ranging from medicine to materials science. Organic chemistry, which studies carbon-containing compounds, is a testament to the incredible diversity and importance of covalent bonds involving carbon.
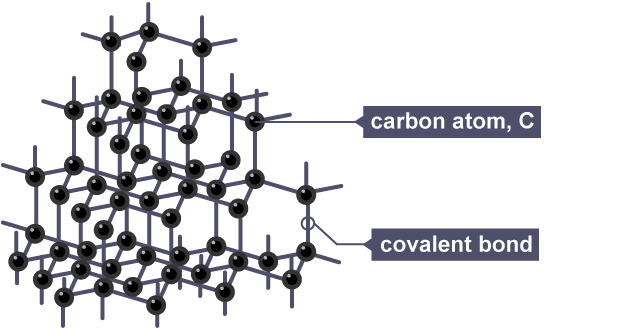
The Stability of Giant Covalent Structures
Giant covalent structures like diamond, graphite, and silicon dioxide are incredibly stable. You'd need a lot of energy to break those solid covalent bonds. Graphite and other carbon forms like graphene are exceptions to electrical conductivity. These structures are also used in various industrial applications, from construction materials to electronic components.
(credit: BBC Bitesize)
Two Sides of the Same Coin: Diamond vs. Graphite
Diamond and graphite, composed of carbon atoms, are fascinating in their differences. Diamond is challenging because each carbon atom bonds to four others, forming a robust and rigid structure. In contrast, graphite has a layered structure, making it useful as a lubricant and in pencils. These two forms of carbon demonstrate the range of properties that can arise from different arrangements of covalent bonds.
A Cosmic Perspective on Covalent Bonding
Scientists have discovered a white dwarf star with temperatures so low that the carbon inside has crystallized into a diamond! This discovery extends the relevance of covalent bonding beyond our planet and even our solar system. It also opens up exciting possibilities for research into extreme forms of covalent bonding under conditions we can't replicate on Earth.
Ionic vs. Covalent: What's the Difference?
When metal elements react with non-metal elements, ionic compounds are formed. However, when two non-metal elements react, a covalent bond results. This fundamental difference affects the properties of the resulting compounds, including their electrical conductivity. Understanding this distinction is crucial for fields like materials science, where the type of bonding can dramatically affect a material's properties.
The Math Behind Covalent Bonds
You can determine how many covalent bonds an element will make using a simple rule - take the number eight and subtract the element's group number. Some elements, like phosphorus, can make more bonds than the rule predicts. This rule is a handy shortcut for guessing how elements will bond, which is essential for understanding more complicated molecules and reactions.
Covalent Bonding in Technology
Covalent bonds are not just a subject of academic interest - they have practical applications, too. For instance, our electronic devices' semiconductors rely on covalent bonding to work correctly. That's why understanding these bonds is so crucial for advancements in technology. But that's not all; they are also essential in developing new materials, such as superconductors and nanomaterials, which could one day potentially revolutionize various industries.
Covalent Bonding in Medicine
In the medical field, covalent bonds also play a critical role. For example, many essential drugs are designed to interact with biological molecules through covalent bonding, which can affect their activity in specific ways. Moreover, research into covalent inhibitors, which form strong bonds with their target molecules, is an area of active research in the quest for more effective medicines. In the future, breakthroughs in incurable diseases may even come along through the power of covalent bonding.
Covalent Bonding: Does It Affect the Environment?
Covalent bonds are also significant in environmental science. While their stability makes materials like plastics durable and rigid, it also creates plenty of challenges regarding waste management and recycling. However, understanding these bonds could also lead to better solutions in the future. Confused how? Well, research is continually underway to develop enzymes that can break down stable covalent bonds, making plastics easier to recycle.
Additionally, covalent bonds are essential when creating biodegradable natural fibres such as cotton. They're also crucial in renewable energy technologies, such as organic solar cells and greenhouse gas capture. So, while it's true that covalent bonds can present many environmental challenges, they can also be vital in creating solutions for these problems!
Conclusion
Covalent bonding is a foundational force that influences everything from the water we consume to the diamonds we treasure. These bonds provide stability and versatility and affect many structures, from simple molecules to complex materials. Their significance isn't confined to our daily lives either - they also have implications in cosmic phenomena. Unlike ionic bonds, which involve the transfer of electrons, covalent bonds share electrons, resulting in stable, neutral molecules with distinct properties. To sum it up, covalent bonding is the unseen force that holds together our world and the universe.
Summarise with AI:












Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic