In previous topics, you learned about matter and its different types. Now let's proceed with changes in the state of matter. Yes, you read it correctly, matter can change its form. You can turn solid into liquid and liquid into gas and vice versa. This topic requires separate attention and that is why we made a separate resource regarding changes in the state of matter. Let's begin, shall we?

How Changes Occur?
To understand this phenomenon, let's do a small experiment. Get an ice cube and place it on a plate. After some time, you will see that the ice cube disappeared into liquid. In the world of chemistry, we see that the solid has turned into liquid. The matter changed its state from solid to liquid. Another example is the boiling of water. When you boil the water, you will see steam releasing from the top of the bubbling water. What is happening is that liquid is turning into gas, slowly.
In simple words, matter can change its state under certain conditions. How is this possible? The kinetic particle theory has the answer to this mystery. According to this theory, particles are in motion, the motion depends on the energy that each state has. Solids have less motion energy and gas have the highest motion energy. When you provide energy to the solids, the particles in the solid absorb the energy and the vibration became so intense that they start to move. When particles start to move that means the state is changing from solid to liquid and if you provide more energy, the particles will absorb more energy hence they will move faster and freely, which is the same condition of particles in the gas. That is how changes occur in states, energy is the driving force behind state changing.
Let's do the opposite, if you take energy from the gas, the particles will lose their energy hence the free movement will turn into less movement just like the motion of particles in the liquid. And if you keep taking more energy, a point will come when the particles will stop moving and start vibrating like solids. That is the maximum point achievable, you can't make solid particles stop vibrating. Hence, the cycle can turn either side depending on the energy, whether you are providing energy or taking it away.
At this point, you might be wondering what is that energy that is involved in the change of state? The answer is heat energy. Remember the above examples? The ice cube is taking heat energy from its surroundings. The boiling water is getting the heat energy from the stove. Therefore, heat energy is the driving force behind the movement of particles.
Each transformation process has its own specific name and these names are to be memorized properly.
Melting
Melting is the process of turning the solid state into the liquid state. Let's create a scenario that we will discuss in all other transformation processes. Imagine a block of ice, the block of ice acquires heat from the surrounding. The heat energy caused the particles, in the solid, to vibrate so frequently that they start to move. Time by time, the energy will be absorbed by more particles causing them to move. Basically, the heat energy is too much that it overcomes the forces of attraction. The particles break their fixed positions and start moving, however, the movement is not too freely. The particles are moving but in their fixed position.
You might be thinking about how to measure the heat energy required for melting? Then the answer is temperature. The temperature of the ice cube is 0°C which means at this temperature, the water will be ice and if you increase the temperature, the ice will start to melt and a time will come when the ice block will turn into liquid.

Freezing
In melting, we turned solid into liquid but in freezing, we turn liquid back to solid by taking the heat energy from the particles. The liquid you received from the ice is at 6°C. You need to remove the heat energy to lower the temperature back to 0°C for the liquid to turn back to solid ice. You place the water in a refrigerator to lower its temperature. When the temperature reaches 0°C, the water particles will have very little heat energy causing them to vibrate only. Therefore, all the particles are packed in a fixed position.

Boiling
Let's bring our liquid back. For some reason, you collected the water and started heating it until you see the vapours of the liquid. This process is called boiling. In this process, the matter changes its state from liquid to gas. You need to provide heat in order to change the state. Water boils at 100°C and when this temperature is achieved by the liquid water, it turns to gas. When you boil a liquid, it turns to gas. The driving force behind this change is heat energy. When heat energy is absorbed, particles begin to move faster hence the force of attraction becomes very low and particles move freely without any fixed position. The particles can move freely anywhere they want in a gas state.

Condensation
When the water was boiling, it was converting into gas but you accidentally put a lid on it. After some time, you remove the lid, you saw water droplets stick to the surface. The question is, when you were heating the water, it should have turned fully into gas but that didn't happen? How? Let's understand the chemistry behind it. When the vapours came in contact with the lid, the lid starts to heat up and the heat source was the vapours. The water vapours transferred the heat energy to the lid and that means the heat energy is been lost and that will cause particles to move less freely, just like in the liquid state. The temperature of water vapour decreases from 100°C and the state changed from a gas back to liquid. This process is called condensation.
Condensation is the process in which gas turns back to liquid by losing heat energy. When the gas loses its heat energy, the particles lose their moving energy (kinetic energy) and they stop moving as freely as they use to do before due to the force of attraction.

Sublimation
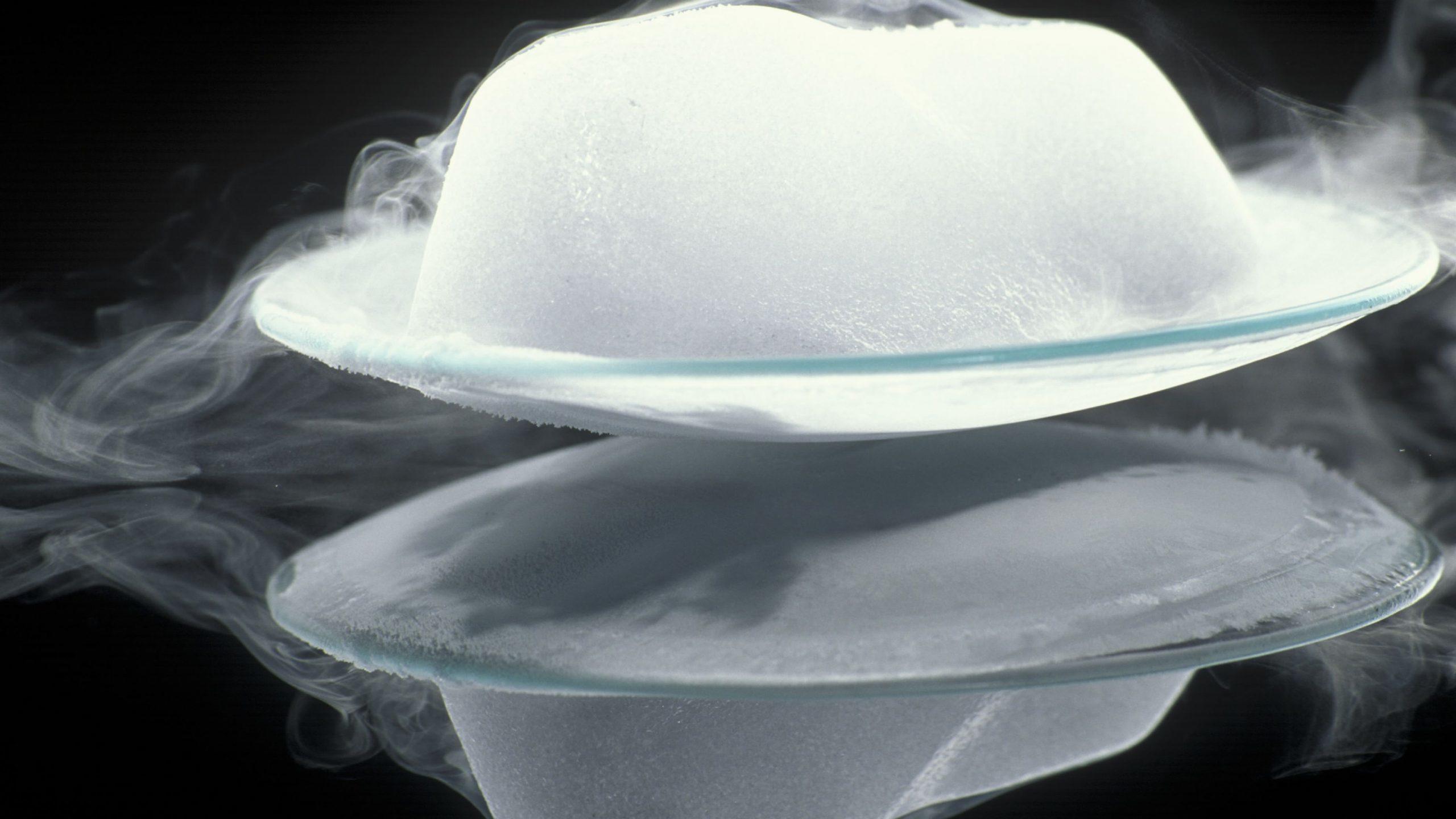
Ever been to a concert? Then you might have seen the artificial fog that is added for the drama effect. That artificial fog is the gas formed from solid carbon dioxide. We call it dry ice. The temperature of dry ice is -78°C and above this temperature, the solid carbon dioxide converts directly to carbon dioxide gas. The conversion of solid to gas is so fast that carbon dioxide doesn't melt, it directly turns to gas. This process is called sublimation.
Sublimation is a process in which a solid turns to gas directly without going through the melting process. This occurs because the atoms on the surface of the solids have so much energy stored within them that when they start to eject from the surface, the high energy allows the atom to eject at high speed just like gas. Iodine and ammonium chloride are two common examples of solids that sublime.
Summarise with AI:












Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic