Chapters

June 2019 - Paper 1 Foundation
01 This question is about atomic structure.
Figure 1 represents an atom of element Z.
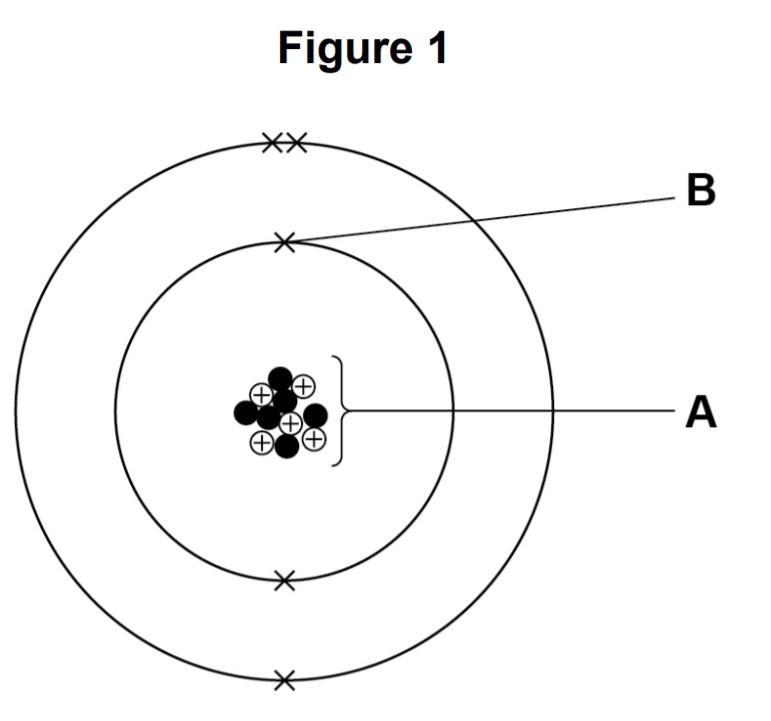
Name the parts of the atom labelled A and B.
Choose answers from the box.
Answer: The A part is the nucleus and the B part is an electron.
01.2 Which particle has the lowest mass?
Choose the answer from the below.
electron neutron nucleus proton
Answer: Electron has the lowest mass as compared to all other subatomic particles. The mass of an electron is 1/1840.
01.3 Which group of the periodic table contains element Z?
Use Figure 1.
Answer: Group 3 because it contains 3 electrons in its valance shell.
01.4 Give the atomic number and the mass number of element Z.
Use Figure 1.
Choose answers from the below numbers.
1 5 6 11 16
Answer: The atomic number is 5 and the mass number is 11. The atomic number is the same as the proton number which can be counted from the diagram. The positive circles are the protons and if you count them, you will learn there are 5 protons. The mass number is the sum of neutrons and protons. The black dots are the neutrons and if you count them, they are 6. The mass number is the sum of neutrons and protons hence 5 + 6 = 11.
Bromine has two different types of atom.
The atoms have a different number of neutrons but the same number of protons.
01.5 What is the name for this type of atom? Tick one box.
Compound
Ion
Isotope
Molecule
Answer: Isotopes because isotopes are the same elements having the same number of protons but different numbers of neutrons.
01.6 The different types of bromine atom can be represented as 79Br35 and 81Br35.
The relative atomic mass (Ar) of bromine is 80.
Which statement is true about the number of each type of atom in bromine?
Tick one box.
A There are fewer 79Br35 atoms than 81Br35 atoms.
B There are more 79Br35 atoms than 81Br35 atoms.
C There are the same number of 79Br35 atoms and 81Br35 atoms.
Answer: The answer will be C because the number of atoms will only change if you change the proton number. In the case of bromine, the neutron number is changing, not the proton number hence both have the same number of atoms but with different weights.
02 This question is about compounds of oxygen and hydrogen.
Figure 2 represents the structure of hydrogen peroxide.
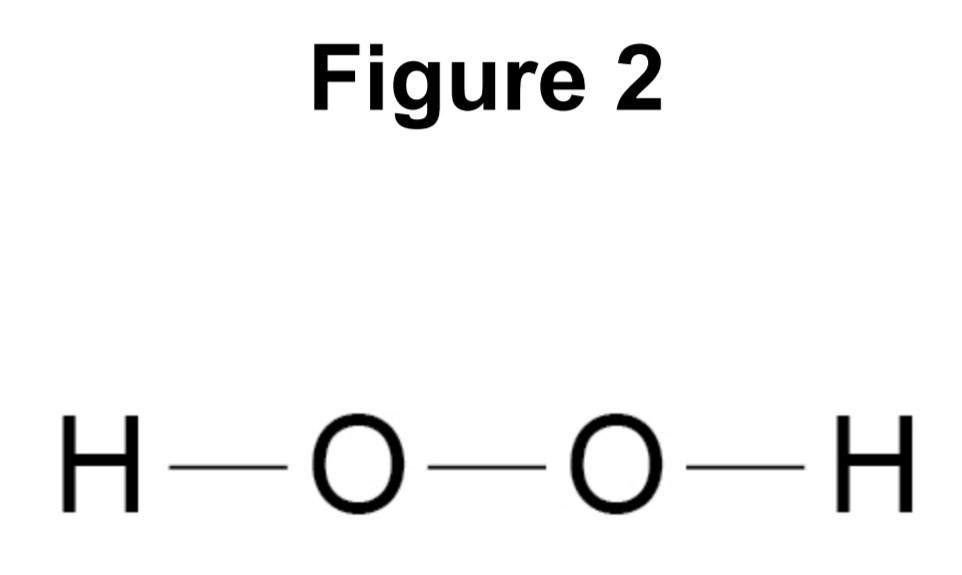
What is the correct formula of hydrogen peroxide?
Tick one box.
H2O2
HO2
H2O2
H2O2
Answer: Part D is the correct answer because there are two atoms of hydrogen and oxygen. This number is denoted by the subscript in a chemical formula. Therefore, A and C options were out of the box. Option B and C have subscripts but D is correct because it shows two atoms of hydrogens and oxygens.
03 This question is about elements, compounds and mixtures.
Figure 5 shows five different substances, A, B, C, D and E.
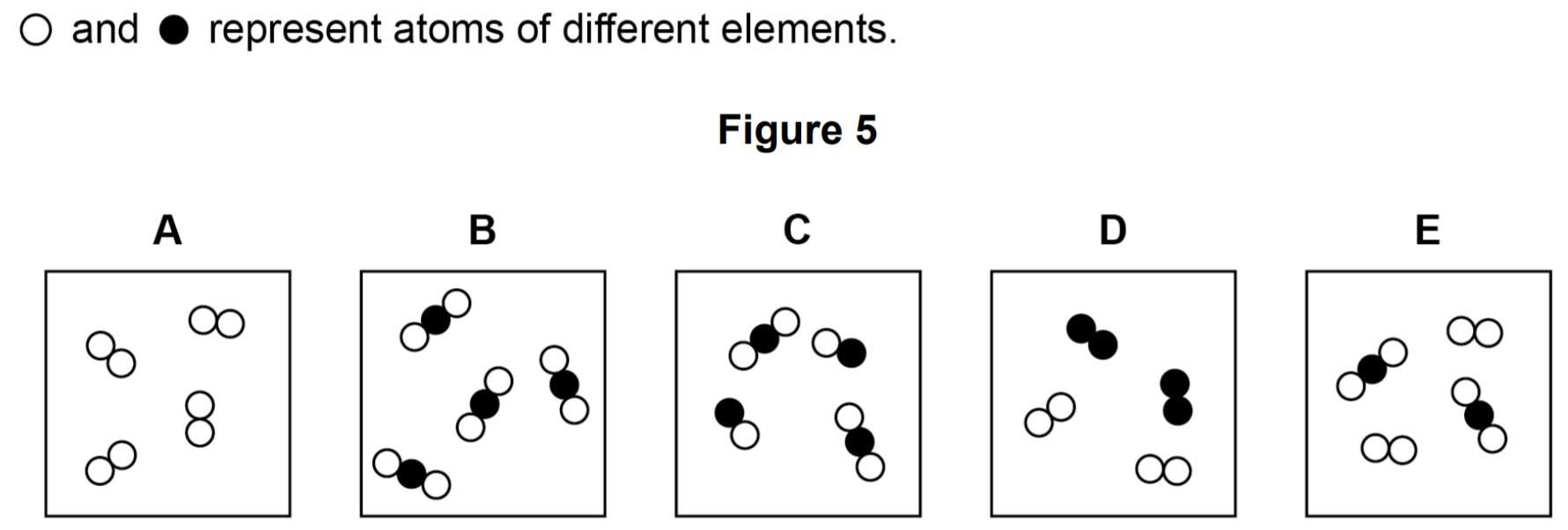
Use Figure 5 to answer Questions 03.1 to 03.3.
03.1 Which substance is only one compound?
Answer: Option B
03.2 Which substance is a mixture of elements?
Answer: Option D
03.3 Which substance is a mixture of an element and a compound?
Answer: Option E
June 2019 - Paper 1 Higher
04 This question is about atomic structure.
04.1 Atoms contain subatomic particles.
Table 2 shows properties of two subatomic particles.
Complete Table 2.
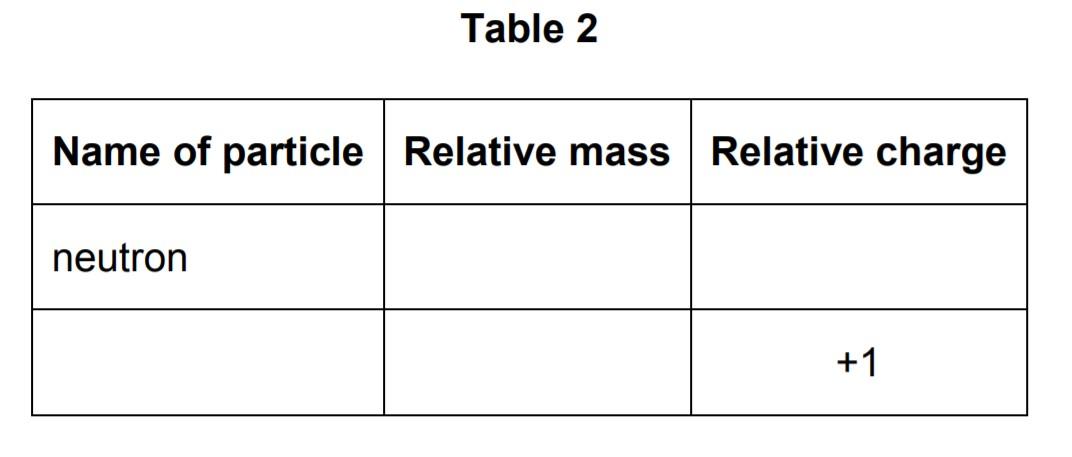
Answer: The relative mass of neutron will be 1 and the relative charge will be 0. The next row will be the proton because only the proton has a relative charge of +1. The relative mass of the proton is 1.
An element X has two isotopes.
The isotopes have different mass numbers.
04.2 Define mass number.
Answer: The mass number is the sum of proton numbers and neutron numbers. It is the number that defines the total mass of an atom.
04.3 Why is the mass number different in the two isotopes?
Answer: In isotopes, the number of neutrons is changed. The mass number is the sum of proton and neutrons and since neutron numbers are changed therefore the mass number of isotopes will be changed too.
04.4 The model of the atom changed as new evidence was discovered.
The plum pudding model suggested that the atom was a ball of positive charge with
electrons embedded in it.
Evidence from the alpha particle scattering experiment led to a change in the model of
the atom from the plum pudding model.
Explain how.
Answer: In the alpha particle scattering experiment, the alpha particles were passed through a gold foil. Most of the alpha particles were detected at the end showing that most of the atom is empty. The highest deflection of the particle was observed in the centre shows that the mass of the whole atom is concentrated in the centre which is called the nucleus and it contains positive particles. The remaining particles deflected at an angle that was greater than 1º.
June 2018 - Paper 1 Foundation
01.5 What is a mixture of metals called?
Tick one box.
An alloy
A compound
A molecule
A polymer
Answer: Alloy.
03 This question is about the structure of the atom.
03.1 Complete the sentences.
Choose answers from the box.
Each word may be used once, more than once, or not at all.
Electron, Neutron, Proton, Ion, Nucleus
The centre of the atom is the .................................
The two types of particles in the centre of the atom are the proton
and the .................................
James Chadwick proved the existence of the .................................
Niels Bohr suggested particles orbit the centre of the atom. This type of particle
is the .................................
The two types of particles with the same mass are the neutron
and the .................................
Answer:
The centre of the atom is the Nucleus.
The two types of particles in the centre of the atom are the proton
and the Neutron.
James Chadwick proved the existence of the Neutron.
Niels Bohr suggested particles orbit the centre of the atom. This type of particle
is the Electron
The two types of particles with the same mass are the neutron
and the Proton.
Table 2 shows information about two isotopes of element X.
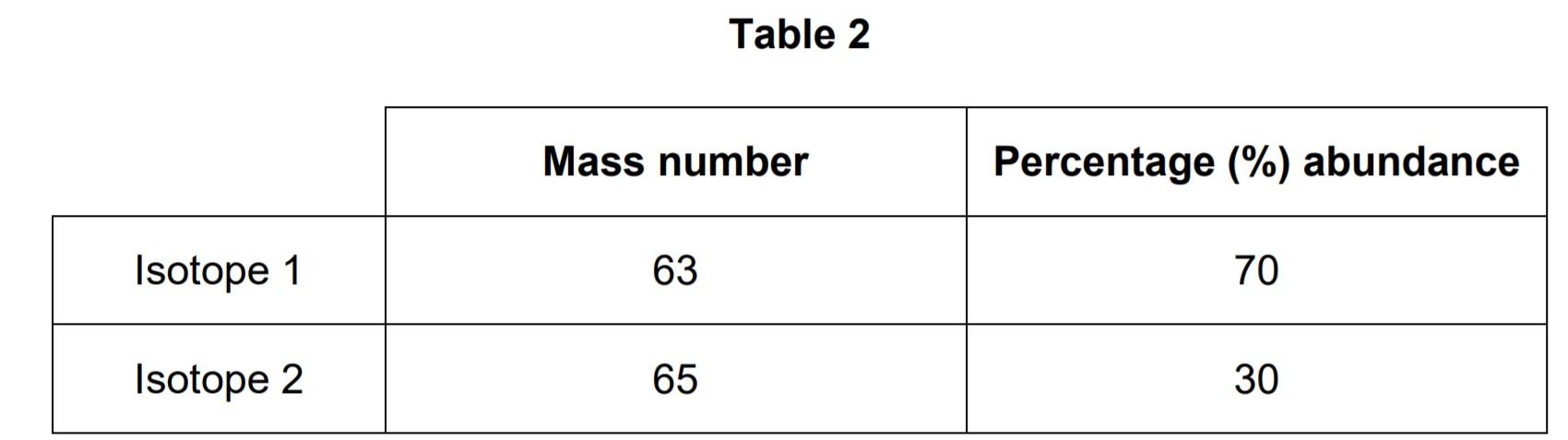
03.2 Calculate the relative atomic mass (Ar) of element X using the equation:
Ar= (mass number × percentage) of isotope 1 + (mass number × percentage) of isotope 2 / 100
Use Table 2.
Give your answer to 1 decimal place.
Answer: Ar = (63 x 70) + (65 x 30) / 100 = 63.6
03.3 Suggest the identity of element X.
Use the periodic table.
Element X is ______________________________
Answer: Copper.
09.2 An atom of iron is represented as 56Fe26
Give the number of protons, neutrons and electrons in this atom of iron.
Number of protons ______________________________
Number of neutrons ______________________________
Number of electrons ______________________________
Answer: Number of protons = 26
Number of neutrons = 30
Number of electrons = 26
June 2018 - Paper 2 Foundation
01.6 Draw one line from each of the compounds to the formula for the compound.
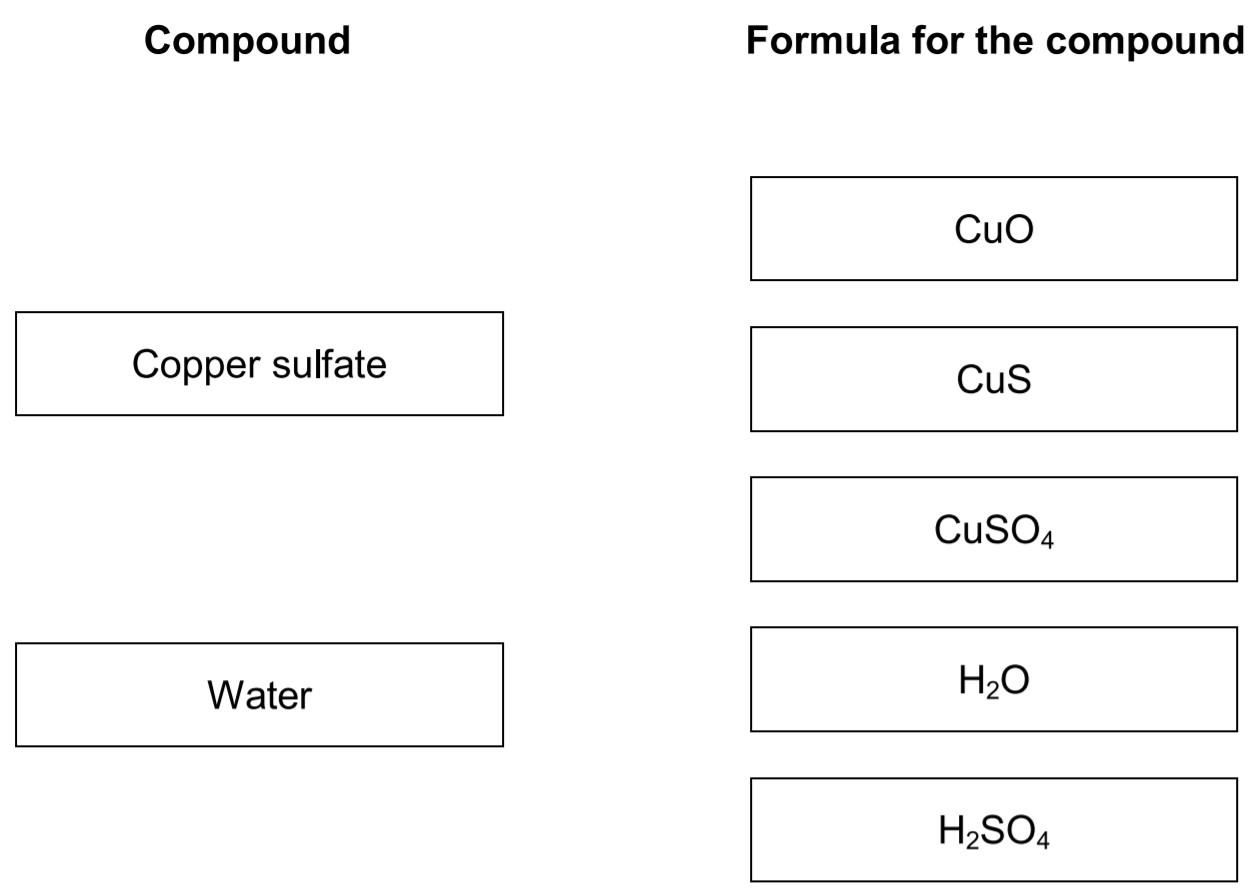
Answer: Attach the copper sulfate with CuSO4 and attach water with H2O.
Summarise with AI:












Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic