Chapters
Do you ever wonder what we all are made up of? We know a physical quantity which is known as mass but what is mass itself? In chemistry, we have a special term for mass, we call it matter. Most of you might know what matter but understanding matter is the building block of chemistry. If you don't know about matter or you have confusion about matter then studying chemistry will be pointless. That is why we decided to make a separate resource about matter and its type and its relationship with the kinetic particle theory.

What is Matter?
One of the frequently asked questions is what is matter? The basic definition is that matter is a substance that occupies space and contains mass. Let's understand the chemistry behind matter. You are reading this blog on your smartphone, laptop, or PC. What do they contain? Electrical circuits, right? Now the next question would be what does electrical circuits contain? They contain atoms. Does your electronic device contain mass? Yes, of course, does your electronic device occupies space? Definitely! That means your electronic device is a matter. Mass and volume are interrelated. Anything that contains mass will always contain volume and that makes it matter.

Forms of Matter
At this point, you have knowledge about matter but matter comes in different shapes and sizes. We call it forms or states of matter. In this world, matter exists in three states and they are:
- Solid
- Liquid
- Gas
At this point, you might be wondering whether the above states are either permanent states or not? Solid, liquid, and gas are three states of matter but they are not something that is permanent. For example, oxygen is usually gas but it can be liquid and solid as well, under certain conditions and those conditions are under special temperature and pressure. Matter can change its state under the given circumstances (i.e. temperature and pressure). We usually consider states under room conditions, which are 22 °C and 1 atm pressure. If you change the temperature or pressure or even both, it can result in a change in the state of matter. For example, you took a glass of water, which is liquid under room conditions. You started boiling the water to make your coffee. You are increasing the temperature of the water but the pressure is not hindered. After some time, the water will turn into a gas at a certain temperature. We will teach you how this happened in the upcoming resources but till here keep it short and simple.
In conclusion, matter exists in three states, solid, liquid, and gas. Matter can change its state according to the given conditions, i.e. temperature and pressure.
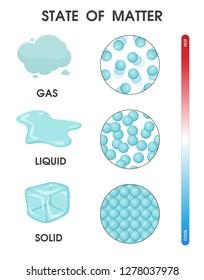
Properties of Solid, Liquid, and Gas
Every state of matter has different properties that make it different from the other states of matter. Each property of the state makes it special and important. Don't worry, there are not too many properties, just 3 main properties that you need to identify the state of matter. We have made a table for that.
| Property | Solid | Liquid | Gas |
|---|---|---|---|
| Shape | Fixed | Not fixed | Not fixed |
| Volume | Fixed | Fixed | Not fixed |
| Compressibility | Cannot be compressed | Cannot be compressed | Can be compressed |
Kinetic Particle Theory
Let's do a small experiment to understand kinetic particle theory. Take a torch and turn off all the lights in your room. Turn on your torch and observe the dust particles that you can see in the beam of light. Do you see the random motion? It looks like they are dancing around but what causes them to dance around? Why they are even dancing? That happens because of air. Air contains very small particles that collide with the dust particles which results in random motion. Now, why can't we see the air particles? Because they are too small for the naked eye to see that is why we can't see those particles but we can detect their presence by observing the random motion of the dust. The phenomena behind the dancing of dust are based on kinetic particle theory.

Sometimes, to understand perfectly, we might give some references from other sciences too such as physics. In physics, kinetic means motion. Any moving object contains a special type of moving energy called kinetic energy and that gives it the name, kinetic particle theory. Both have something common and that is particles in motion but kinetic energy is something that should be discussed in detail so don't get confused, both are different but they talk about one common thing and that is particle being in motion.
So, why do we need kinetic particle theory? Because
- It describes the states of matter;
- It explains the differences in properties of all states of matter;
- It teaches the changes in the state of matter.
Summarise with AI:













Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic