Chapters
Before discovering protons, neutrons, and electrons, scientists tried to classify elements. At that time, atomic number, i.e. proton number, was not discovered. Therefore, scientists tried to classify elements according to their atomic weights. Not many elements were not discovered at that time, yet they tried, and they created a periodic table that was arranged by mass. Later, much development came, leading to today's periodic table, which is very accurate and helps us predict and understand elements. Let's glimpse the past, from the first periodic table to the updated one.

Early Models
In 1864, when sub-atomic particles were not discovered, an English scientist, John Newlands, devised an idea to create a table of elements to keep track of available elements. Since protons, neutrons, and electrons were not discovered at his time, he knew that the atom was the smallest individual particle. He only knows that atoms have different masses, allowing them to classify elements into different atomic masses.
At that time, the objective was to build a table of elements arranged by their atomic mass. When he made the list, patterns emerged. He saw patterns in regular periods along with the list, which showed that elements show similar properties after every eight places ahead. For example, consider lithium (Li) as the first element and the name of the element eight places ahead of it is sodium (Na). Sodium and lithium showed similar behaviours. John Newlands then arranged elements in periods by putting them in vertical columns and calling them groups. This thing gave rise to the term periodic. John Newlands called his whole work the Law of Octaves.
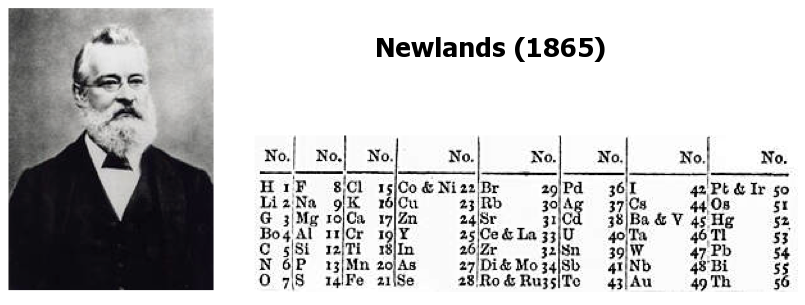
The table had flaws. The repeating pattern was successful initially, but eventually, it failed. The primary focus of the table was to follow the atomic mass, not finding similar properties, which is why he was forced to add some elements that didn't have similar properties. For example, iron (Fe) and oxygen (O). They were placed in the same group. However, oxygen is a gas, iron is a solid, iron is metal, and oxygen is a non-metal. This flaw was unacceptable to other scientists, leading to the rejection of the first periodic table.
However, this concept has not gone to waste. Many other scientists from different countries tried to follow Newland's law but were unsuccessful until 1869.
Mendeleev's Periodic Table
After five years of the Law of Octaves, a Russian chemist, Dmitri Mendeleev, it made a periodic table that was considered the most accurate at that time.
He also made the periodic table according to the increasing atomic mass, but with some changes, his periodic table was acceptable. He observed that the element's physical and chemical properties were periodically related to their atomic mass. Therefore, he arranged them in vertical groups.
Gaps in Mendeleev's Periodic Table
This method of arrangement created gaps in the rows (known as periods). However, Mendeleev didn't see this as a problem but an opportunity. He found that the gap requires a different element to be discovered, which will fill the gaps in the horizontal rows.
This also allowed him to find the atomic mass and predict the properties of the missing elements. Today, the properties of the elements found and fitted in the gap have the same atomic mass and properties as predicted by Mendeleev. Furthermore, Mendeleev switched the order of elements to maintain consistency down the columns.
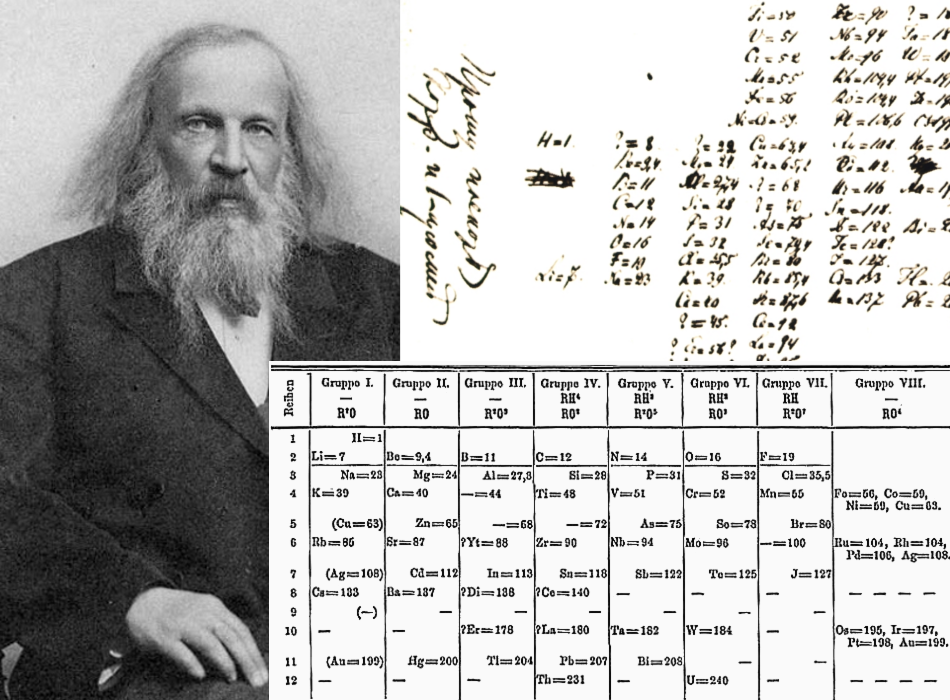
Isotopes
When Mendeleev finished the periodic table, he noted that some elements didn't fit as he required. There were some anomalies in the periodic table, and those anomalies were isotopes. During Mendeleev's time, there was no concept of isotopes, and Mendeleev saw those isotopes as different elements, so he placed them in his periodic table.
This means that no matter how perfect Mendeleev's table was then, there would be inaccuracy. In conclusion, his table was incorrect because of the isotopes, but later on, when sub-atomic particles were discovered, the periodic table was revised again. When proton was discovered, the protons of all elements were calculated, leading to the discovery of isotopes. Later, the periodic table was corrected, all the elements were correctly arranged, and all those anomalies Mendeleev found were correct.
Evaluation of Both Works
Both works were significant for developing the periodic table that we know today. Thanks to them, we can learn many things from the periodic table. Both Mendeleev and Newlands arranged all the elements according to their atomic weight. There were irregularities in the table, but the Newlands first introduced this concept, and Mendeleev corrected his work; for example, Mendeleev swapped the order of some elements to arrange them according to their properties.
Differences and Similarities
There were some similarities and differences in both works. Although we consider both works different, they are similar to some extent. Below is the table that demonstrates the similarities and differences of both works.
| Similarities | Differences |
|---|---|
| Both works arranged elements according to their atomic weight. | Newland's work adds all those elements that were known at that time. Mendeleev left gaps for elements that would be discovered later. |
| The concept of groups is common in both works. | Newland followed a strict order of atomic weights, while Mendeleev swapped the order to fit elements according to properties. |
| All the elements were the same in both tables. | Elements in the group had similar properties in Mendeleev's table, while every eight elements had the same properties in Newland's table. |
| - | Newland's table was rejected because some elements didn't fit properly. Mendeleev's table was accepted because the elements show similar properties in groups. |
Summarise with AI:













Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic