Chapters
Diffusion and evaporation are two different phenomena. Both processes occur in liquids but diffusion also occurs in gas too. Many students have troubles and doubt and that is why we made a separate resource that will discuss both processes in detail.

Diffusion
We all use room freshener spray. When you spray the room freshener, the scent spreads in the whole room, although you sprayed in one direction yet it spread in the room. Ever wonder why? This is because of diffusion, so what exactly is diffusion? When gas particles are released into an environment, the particles move freely to fill up the whole space. This is called the diffusion of a gas.
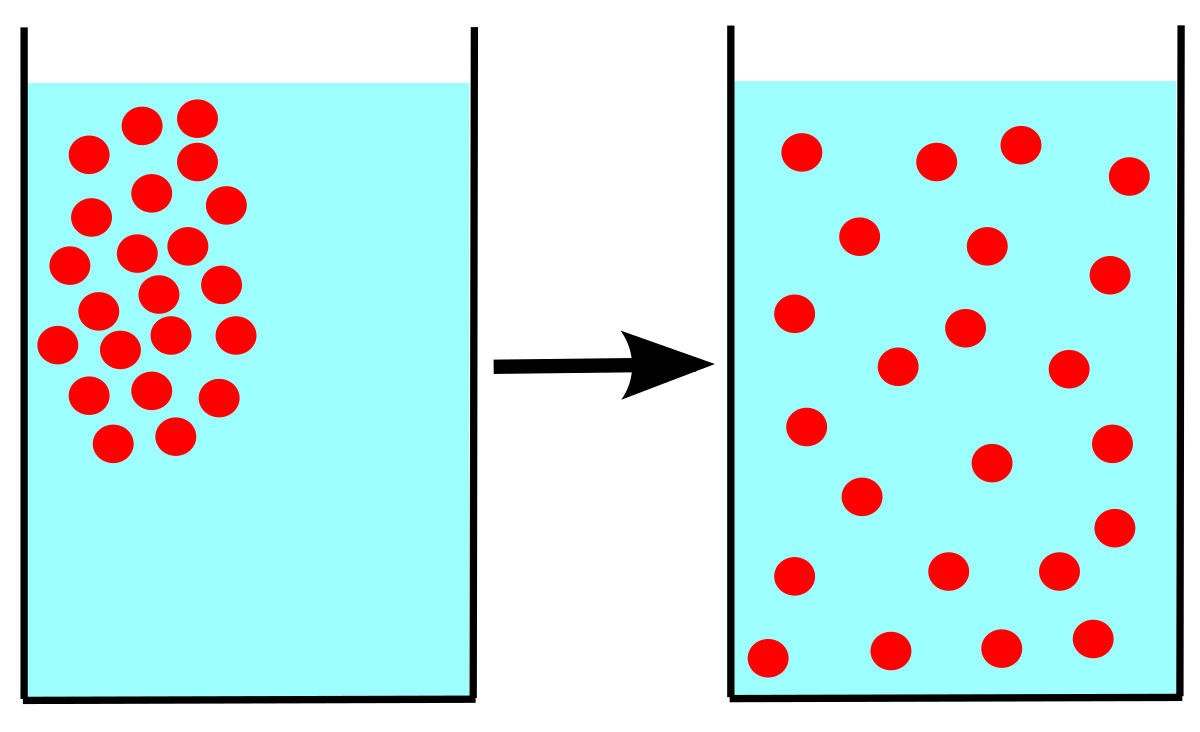
You learned in kinetic particle theory that the particles of gas move freely having no fixed boundaries. When you release gas, it tries to cover the whole place, whether you release gas in a jar or in a room. That is why others are able to smell the scent even if you sprayed in just one corner.
Brownian Motion
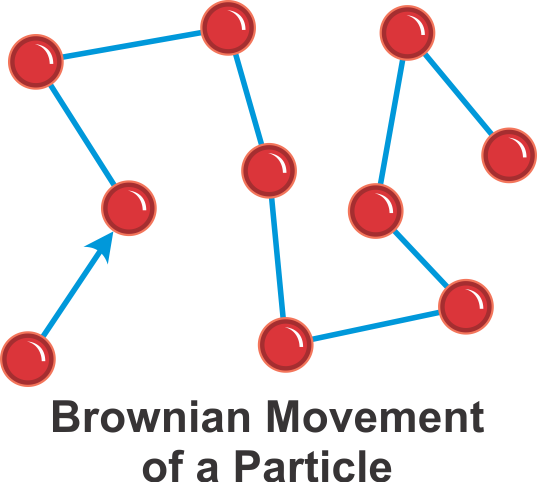
The diffusion phenomena is explained better by the Brownian motion. In this experiment, two jars are taken, one filled with air and the other filled with bromine. Air is a colourless gas while bromine is a brown colour gas. The air jar is placed on the top of the bromine jar but there is a cover between them that is acting as a barrier. When you remove the cover, the bromine gas and air start to mix with each other. A time will come when you will see the whole jar coloured brown. This happened because the bromine gas diffused with the air. If you see the molecule behaviour in the jar, at any point, you will see air molecules and bromine molecules moving in random order. This experiment shows how gases diffuse in each other.
Effect of Molecular Mass on the Rate of Diffusion
At this point, you know how diffusion occurs. Its time to understand how to affect the rate of diffusion. Some gases diffuse slowly while some gases diffuse very fast. For example, hydrogen diffuses very fast as compared to carbon dioxide. How do we find out the rate of diffusion of gas? The answer is in molecular mass.
So, here is the secret, if the molecular mass is low, it will always diffuse faster. This is because weight gases contain fewer molecules and that means they have more space to travel as compared to gas having high weight. More space will allow the gas to move freely and easily while the molecules in other gas will collide with each other due to high traffic.
For example, you have two gas, helium and hydrochloric acid. Which gas will diffuse faster? The answer is helium because the molecular weight of helium is 2, however, the molecular weight of hydrogen chloride is 36.5.
Diffusion in Liquids
Like gas, diffusion also occurs in liquid too. One of the daily life examples of diffusion in liquids is the coffee that most of you drink. When you add coffee to milk, the coffee dissolves in the milk. If we take a look at the molecular level, the coffee molecules diffuse into the space between milk molecules. This is how diffusion occurs in liquids.
To demonstrate diffusion in liquids, fill a beaker with distilled water and add potassium manganate (VII). When you add potassium manganate (VII) to the water, it will settle below and diffusion will take place slowly. Water is a colourless liquid while potassium manganate (VII) colour is purple. You will see two layers, the bottom layer is the potassium manganate (VII) layer and the top layer is the water layer. The water layer will be colourless and the potassium manganate (VII) layer will be purple. After 30 minutes, you will see that the colour of the upper layer will turn purple but not as dense as the bottom layer's colour. After a few hours, you will see that the layers are merged into one and the colour of the whole beaker will turn purple. Let's understand the chemistry behind this.

In liquid diffusion, the molecules move from higher concentration to lower concentration. Therefore in the beaker, the water molecules will move to the potassium manganate (VII) layer and potassium manganate (VII) molecules will move to the water layer. A time will come when both layers will have even molecules of both liquids and that is where the diffusion will stop because the solution is homogenized now. However, one point to be noted, the rate of diffusion of liquids is much slower than the rate of diffusion of gases.
Effect of Temperature on Diffusion
When you provide heat to the gas, the heat energy converts into kinetic energy which causes the molecules to speed up. When molecules travel faster, the rate of diffusion increases because the rate of diffusion depends on the speed of molecules. The faster the molecule travel, the more diffusion. Therefore, increasing the temperature will result in faster diffusion.
Evaporation
Many students are often confused about evaporation and boiling. Both are totally different things but the result of both processes is the same. Let's say you filled a beaker and placed it under the sun. The heat from the sun is in contact with the surface of the water. The molecules on the surface of the water will absorb the heat energy from the sun and they will move more faster and a time will come that they will get enough energy to eject themselves from the water, i.e. turning into gas. However, the bottom layers of water will not get much affected, they will get a little heat up but if we compare the heat energy of both, on the surface and under the surface, the heat energy is maximum at the surface and the heat energy is the least at the bottom.
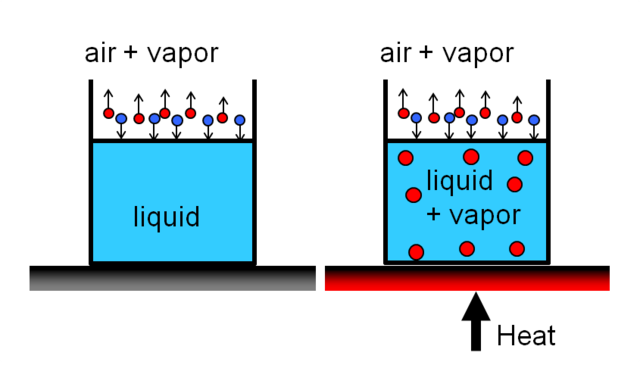
This is the evaporation phenomenon. Evaporation is the surface process and the best part of evaporation is that it can occur at any temperature.
Difference Between Evaporation and Boiling
In both processes, the liquid is turned into gas but with a different methodology. This is a very common question and many students failed to answer it properly.
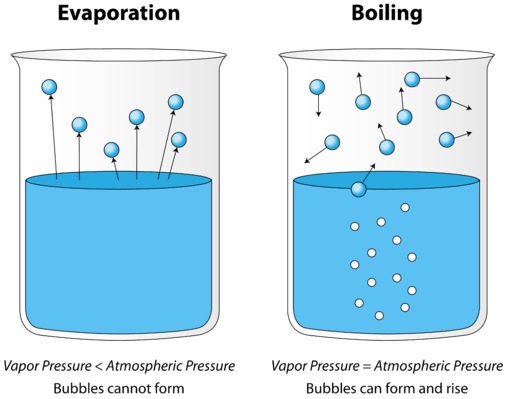
The first difference is that evaporation is a surface phenomenon while boiling involves all the water molecules. Evaporation occurs on the surface of the liquid while boiling involves all the layers of the liquid.
The second difference is that molecules eject from the surface in evaporation while boiling heats the liquid up to its boiling point. When the liquid reaches its boiling point, particles will eject in boiling.
The third difference is that evaporation doesn't depend on the temperature while boiling depends on the temperature.
The fourth difference is that evaporation is a slow process while boiling is a fast process.
Last but not the least, the fifth difference is that once evaporation is done, it produces a cooling effect but this doesn't happen in boiling.
Summarise with AI:













Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic