Chapters
Till here, you have a good concept of matter and its states. Allow us to give you a small recap before we move on. Matter is any object that contains mass and occupies space. Mass and volume are interrelated. Anything that has mass will also occupy space. Matter can be distinguished into three states, solid, liquid, and gas. Each state has its own properties and that makes the state special. However, we never discussed the states in detail. There is more to learn about the different states of matter and that is why we made a separate resource to discuss it. Let's begin, shall we?

Solid
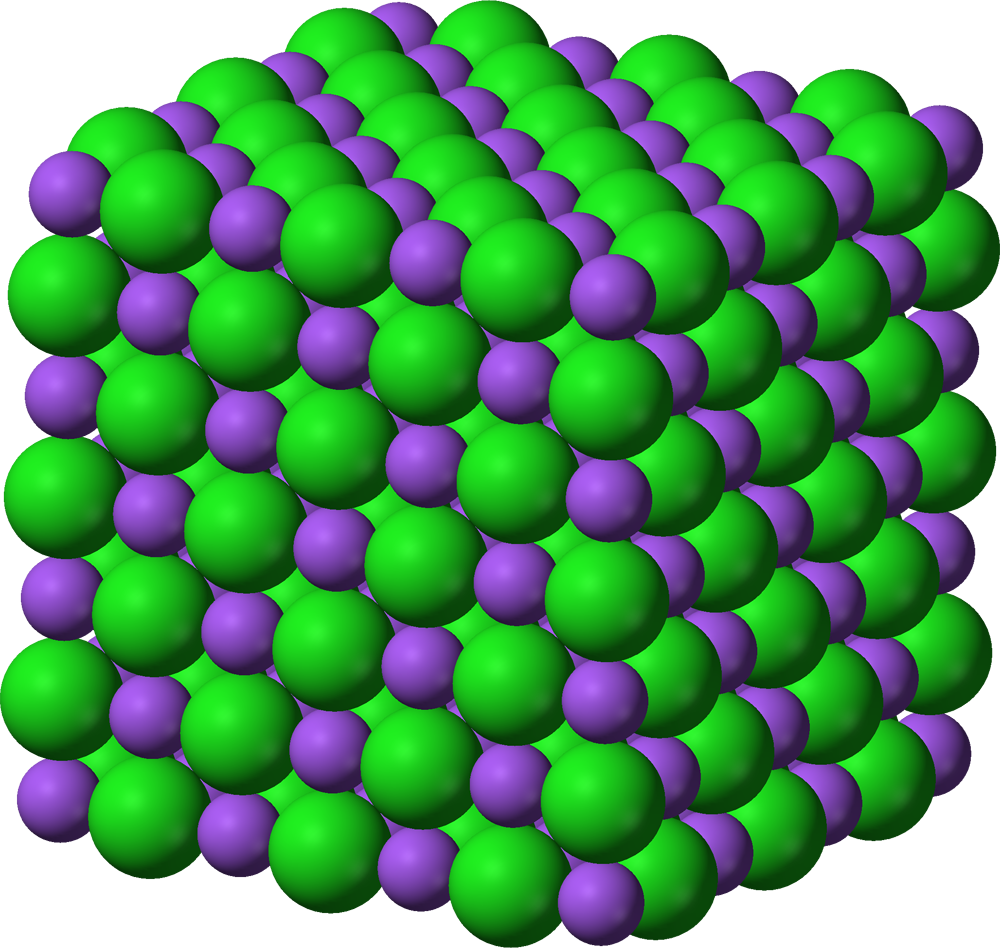
You might be viewing this resource from your smartphone, tablet, or laptop. We discussed that they all are matter but we didn't discuss the state of the matter. Allow us to explain, your laptop, tablet, even your smartphone come in the category of solids. Any object that has a fixed shape will always be solid. The question is, how come solids have a fixed shape? To answer that we need to take a deep look at your laptop, at a molecular level (atomic level). Matter consists of atoms, the states are the arrangement of the atoms. In solids, the arrangement of the atoms are tightly packed. In simple words, they are in an orderly manner which is why solids have specific shapes. Not to mention that they have fixed volume too but solids can't be compressed.
For every state of matter, kinetic particle theory is followed. The kinetic particle theory explains the atomic structure and arrangement of solids. Below are the points that kinetic particle theory explains for solids.
- The particles are closely packed in an orderly manner.
- The particles are held together thanks to the strong forces of attraction.
- The particles in a solid contain kinetic energy that allows them to vibrate only. Particles rotate about their fixed positions too.
- The particles can never move freely, they can only vibrate and rotate but never ever move freely.
Liquid
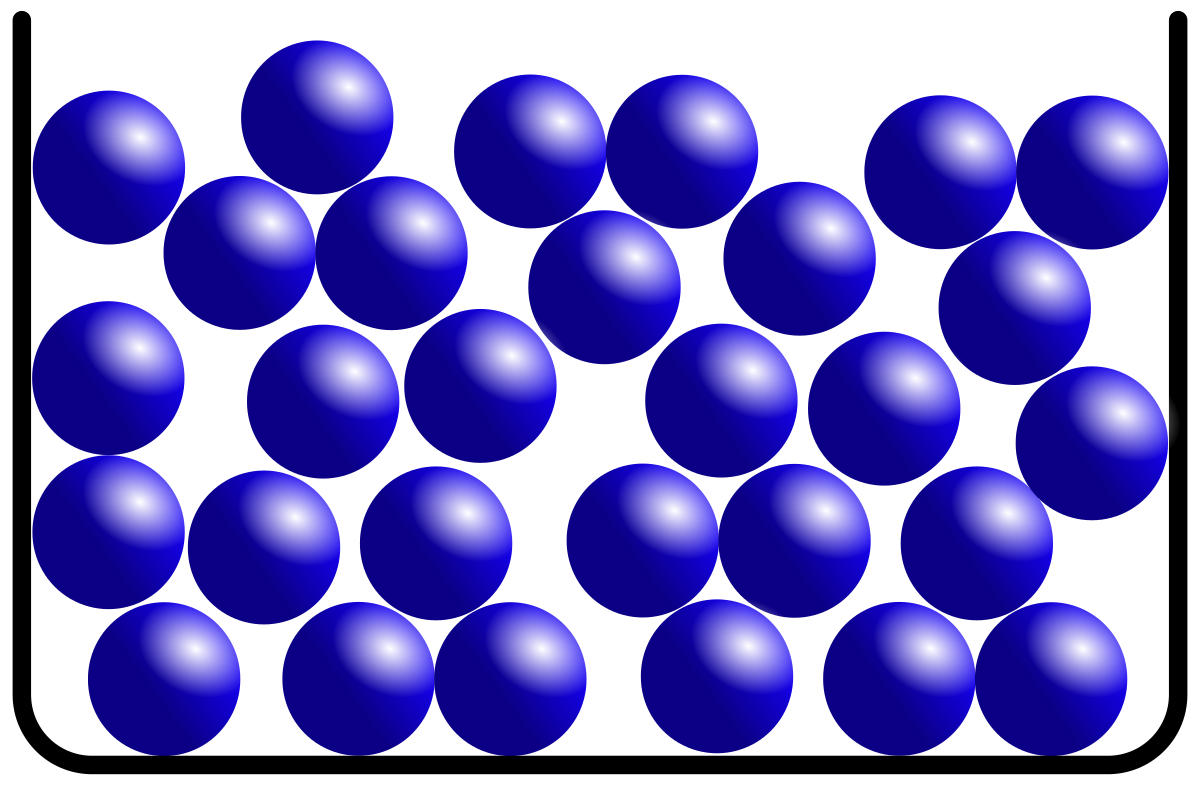
Another state of matter is liquid. One of the biggest examples of liquid is the water that you use in daily life. We all know what water is and that is why we are going to ask you a question, does water has its own shape like solids? Of course not, water doesn't have any shape because it has the ability to flow. Every liquid has the ability to flow and that is why they don't have a specific shape, they take shape of the object that they are placed in. For example, if you pour water into a container, the water will take the shape of that container. You pour water into a mug, the water will take shape of the mug. Hence, liquids don't have a specific shape but they can take shape of the object that they are placed.
You might be wondering how liquids can flow then the answer is in the molecular structure of the liquid. The atoms in the liquid are not in a fixed position. This is because the atoms of liquids can move from one point to another because of the availability of free space between particles. Unlike solids, the arrangement of the atoms is in a disorderly manner. That is why liquids don't have a fixed shape.
However, liquids contain fixed volume. For example, where ever you place the water, the volume of water will never change. You can measure its volume too using measuring instruments. The reason behind fixed volume in liquids is that the particles are far apart from one another. However, they are still packed closely but there are spaces between atoms that allows them to move. That is why liquids cannot be compressed but they have fixed volume.
Let's hear what kinetic particle theory has to say about liquids:
- The arrangement of particles in a liquid is disorderly mannered.
- The forces of attraction in liquids are low compared to solids.
- Particles in liquids contain more kinetic energy than solids, allowing the particles to move.
Gas
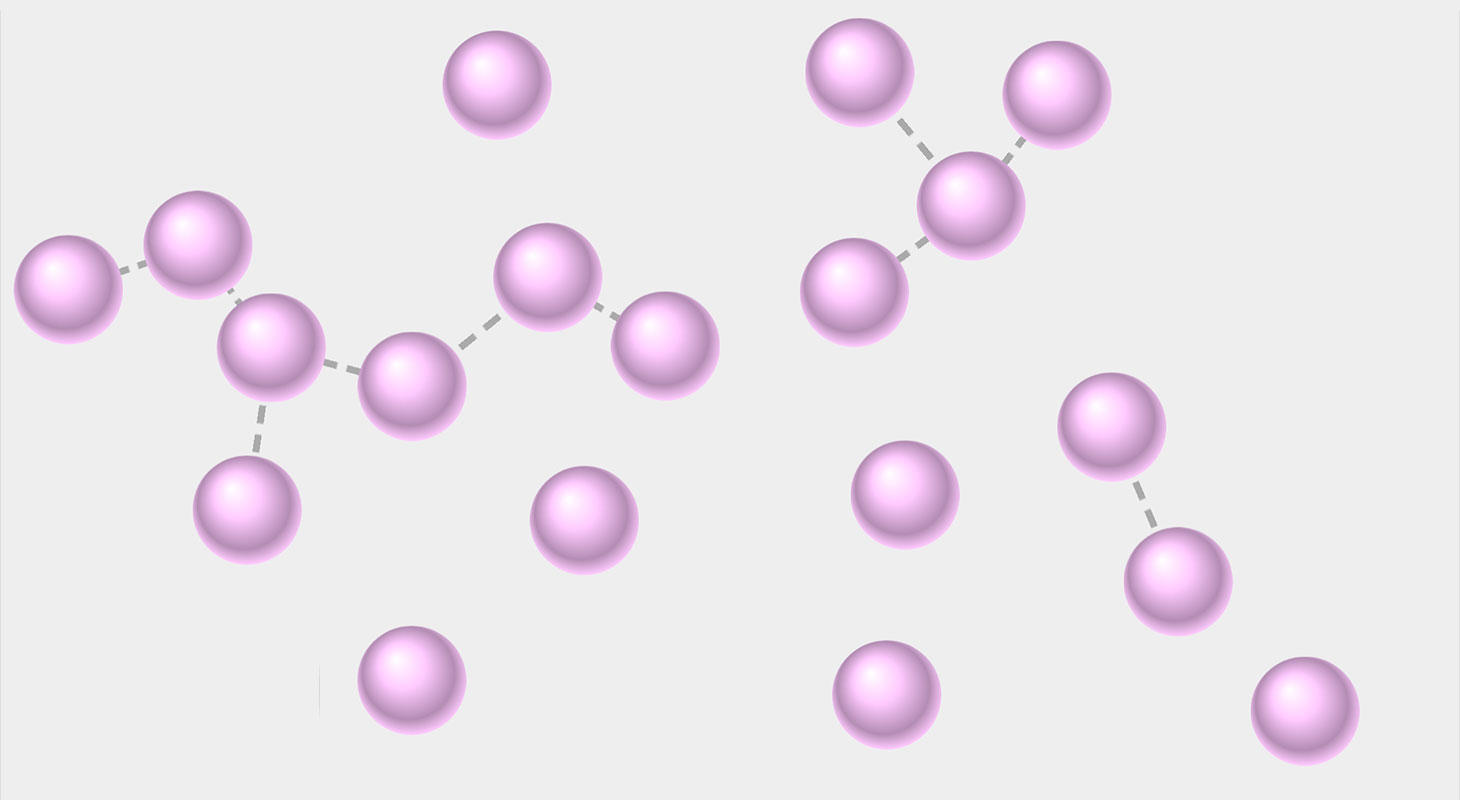
Last but not least, gas is the 3rd state of matter. The invisible air around us is a perfect example of gas. In fact, your breath is also a gas. Like liquids, gas can flow too but have you ever seen gas spreading? One of the famous experiments relating to gas spreading is the Brownian motion. In this experiment, brown gas is mixed with ambient gas and you see how they are mixing. This phenomenon is called diffusion which we will discuss in upcoming resources. This property of gas allows it to be different and wonder why? Because of its particles. The particles in a gas are so far apart that they move freely because of the vacant space between particles. This also allows gas to be easily compressed. Like liquids, gas doesn't have a shape but it takes the shape of the container.
An additional point that differentiates gas from a liquid is that gas doesn't have a fixed volume like liquids have. This is because the space between particles is enormous which allows gas to be compressed under pressure. The compression will result in particles moving closer and this means volume is changing. That is why gases don't have a fixed volume.
The kinetic particle theory says that:
- Particles in a gas are spread far apart.
- Particles have weak forces of attraction as compared to solids and liquids.
- Particles contain high kinetic energy which allows them to move freely and they are not held in fixed positions like in solids.
Conclusion
Solid, liquid, and gas are three states of matter. Each state is different from the others. The biggest difference is in the atomic arrangement. According to their arrangement of atoms, their physical properties change. In fact, the whole kinetic particle theory is about the arrangement of particles. This is the fundamental chemistry that every student should know in order to understand complex chemistry which will come sooner than you think.
Summarise with AI:












Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic