Chapters

Understanding Atomic Structure
Atoms are the building blocks of everything around us — from the air you breathe to the device you’re reading this on. But what exactly is an atom made of, and how do scientists represent it? Let’s explore the atomic structure step by step, with simple explanations and diagrams to help you visualise what’s going on inside.
What Is an Atom?
An atom is the smallest unit of matter that can take part in a chemical reaction. It’s incredibly tiny — you can’t see it with the naked eye or even a normal microscope. Scientists use powerful tools, such as scanning tunnelling microscopes, to observe atoms.
Although atoms are unimaginably small, they have a well-defined internal structure.
The Three Subatomic Particles
Every atom is made up of three main particles:
| Particle | Charge | Location | Relative Mass |
|---|---|---|---|
| Proton | Positive (+) | Nucleus | 1 |
| Neutron | Neutral (0) | Nucleus | 1 |
| Electron | Negative (–) | Electron shells | ~0 |
At the centre of the atom lies the nucleus, containing protons and neutrons. This dense core makes up almost all the mass of the atom. Surrounding the nucleus are electrons, which move around in energy levels or shells. Electrons have very little mass but are crucial because their arrangement determines how atoms react with one another.

Electron Shells and Energy Levels
Electrons occupy shells (also called energy levels) around the nucleus. Each shell can hold a specific number of electrons:
| Shell | Maximum Electrons |
|---|---|
| 1st (K shell) | 2 |
| 2nd (L shell) | 8 |
| 3rd (M shell) | 8 |
| 4th (N shell) | 18 |
Electrons fill the lowest energy shell first before moving to the next.
How to Draw an Atomic Structure
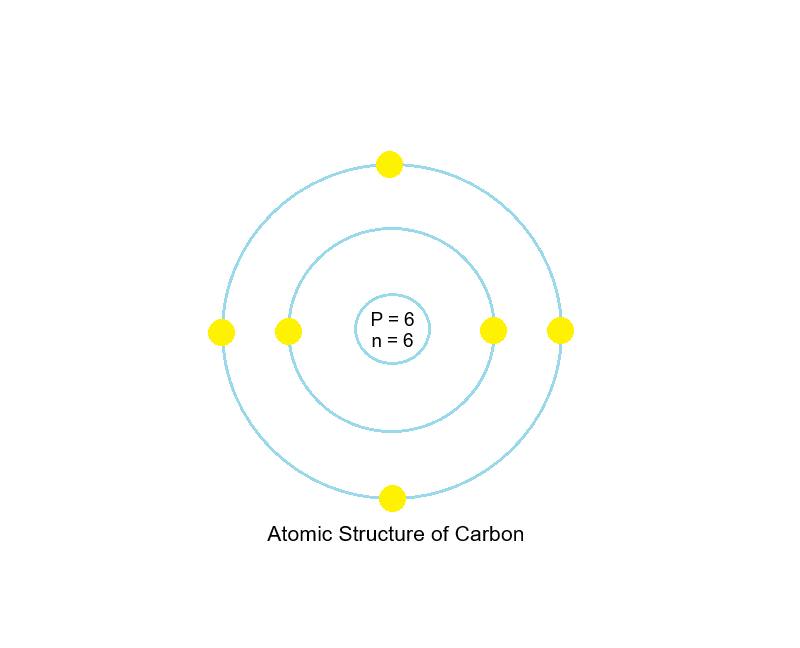
Let’s go step-by-step with an example — Carbon (C), which has 6 protons and 6 electrons.
Step 1: Draw a small circle for the nucleus.
Label it with the number of protons (6p) and neutrons (6n).
Step 2: Draw the first shell — it holds 2 electrons.
Step 3: Draw the second shell — it holds the remaining 4 electrons.
Result:
Carbon’s electronic configuration is 2, 4.
This means:
- 2 electrons in the first shell
- 4 electrons in the second (its valence shell)
Electronic Configuration Explained
The electronic configuration shows how electrons are arranged in shells. It’s written as a series of numbers separated by commas.
| Element | Atomic Number | Electronic Configuration | Valence Electrons |
|---|---|---|---|
| Hydrogen (H) | 1 | 1 | 1 |
| Carbon (C) | 6 | 2, 4 | 4 |
| Oxygen (O) | 8 | 2, 6 | 6 |
| Calcium (Ca) | 20 | 2, 8, 8, 2 | 2 |
1. An atom has protons, neutrons, and electrons
2. Protons (+) and neutrons (0) are in the nucleus
3. Electrons (–) move in shells around the nucleus
4. Shells fill in order: 2, 8, 8, 18...
5. The valence shell determines reactivity
Why Understanding Atomic Structure Matters
Knowing how atoms are built helps explain:
- Why elements form compounds
- Why some elements react violently while others are stable
- The entire Periodic Table arrangement
From chemistry to physics, this tiny structure shapes everything in science.
Summarise with AI:












Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic