Chapters
Covalent compounds are an integral part of our daily lives, present in everything from the water we drink to the molecules that make up living organisms. Interestingly, these compounds are formed when atoms share electrons, creating a stable and often less reactive molecule. But how does this fascinating process work, and how can you make covalent compounds yourself? Let's take a magnifying glass to this captivating subject.

Covalent Compounds: What are they?
Before we get into the nitty-gritty, it's crucial to understand what covalent compounds are. Simply put, a covalent compound is formed when two or more nonmetal atoms share electrons. This sharing helps the atoms achieve a stable electron configuration, often resembling a noble gas. Due to this, covalent compounds are generally more stable and less reactive than ionic compounds, which are formed through the transfer of electrons.
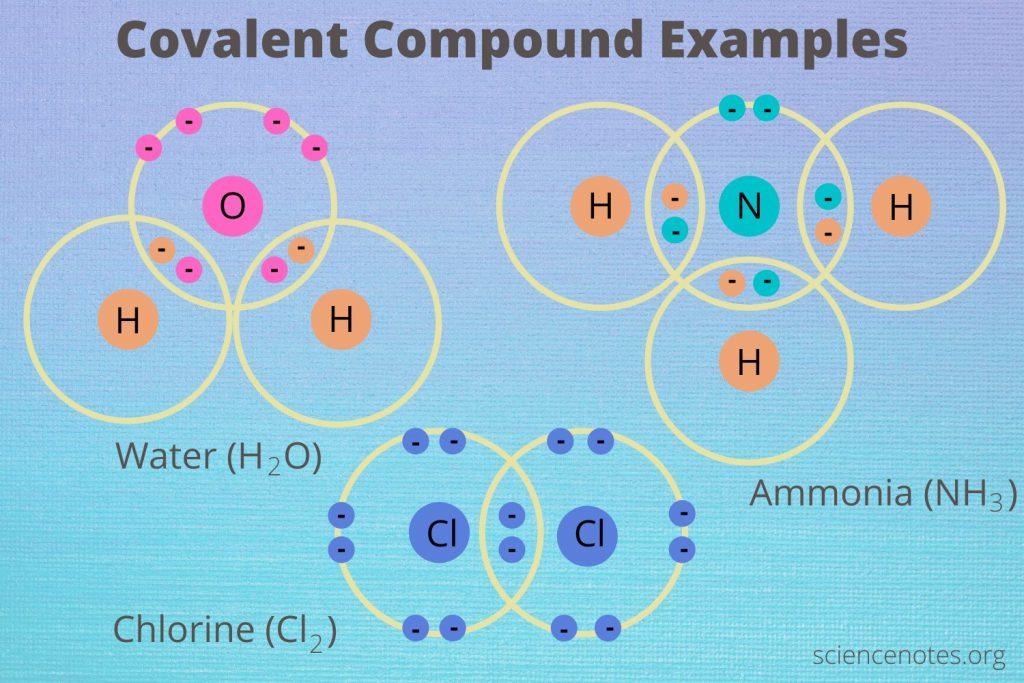
Identifying the Right Elements
The first step in making a covalent compound is identifying the elements that can form one. Typically, nonmetals are your go-to element for creating covalent bonds. For example, elements like carbon, oxygen, nitrogen, and sulfur are commonly used to form these compounds. Hydrogen, although not a nonmetal, can also form covalent bonds with nonmetals.
The Importance of Valence Electrons
Valence electrons are the key players in forming covalent compounds and are the electrons in the outermost shell of an atom. Atoms aim to fill or empty this outer shell to achieve stability; sharing electrons through covalent bonding is one way to do it. Therefore, knowing the number of valence electrons in your chosen elements is crucial for successful bonding.
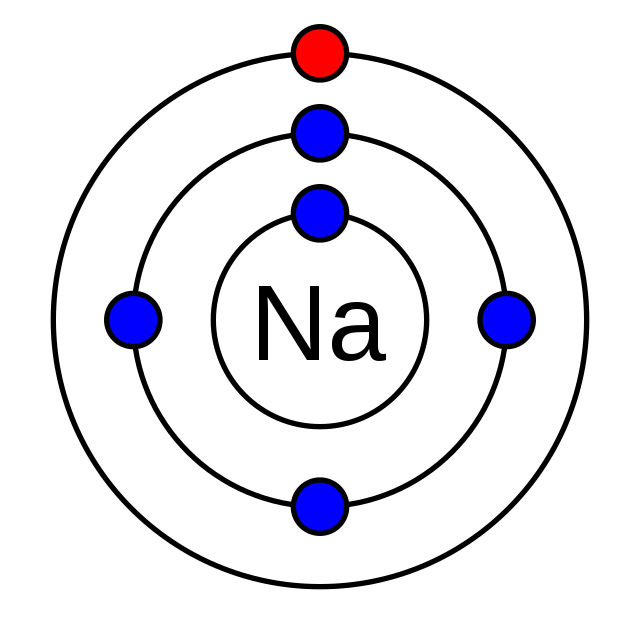
The red dot represents the electron's position in the shell of an atom (credit: Chemistrytalk)
Making Covalent Compounds: A Basic Guide
Creating a covalent compound involves several key steps. First, you'll need to choose the nonmetal elements you wish to combine and ensure they can form covalent bonds. From here, write down the symbols of these elements to help visualize the compound and plan the bonding process. Next, look at the periodic table and count the valence electrons for each element, which will help you know how many electrons need to be shared for stability. After this, you can draw Lewis dot structures or use molecular models to visualize how the atoms share electrons. Finally, confirm that the outer electron shells of all participating atoms are whole. If they are, congratulations! You've successfully created a covalent compound.
How Scientists Create Covalent Compounds in the Lab
After identifying the correct elements and understanding their valence electrons, scientists are ready to move to the practical phase of creating covalent compounds. Here's how they typically go about it in a laboratory setting.
Preparation: Scientists will gather the needed elements and lab equipment, including a precision balance for accurate measurements.
Safety: Protective gear like gloves and goggles are worn, and work is often done under a fume hood for safety.
Weighing: The elements are precisely weighed based on the compound's stoichiometry.
Mixing: The weighed elements are combined in a test tube or beaker. A solvent is added if needed.
Temperature Control: Depending on the compound, the mixture may be heated or cooled using Bunsen burners or ice baths.
Stirring: A magnetic stirrer or glass rod is used to mix the elements, facilitating covalent bonding.
Reaction Time: The mixture may need to stand for a while to allow bonds to form.
Monitoring: Scientists monitor the reaction for signs like a colour change or a gas release.
Testing: After the reaction, the compound is verified through tests like melting point checks or advanced techniques like NMR.
Purification: Finally, the compound is purified using methods like filtration or distillation.

Real-world Examples
Understanding covalent compounds is easier when you can relate them to real-world substances. Below are some examples to highlight how varied these compounds are.
Carbon Dioxide (CO2): One carbon atom forms double bonds with two oxygen atoms in carbon dioxide. This fills the outer shells of both atoms, making it a stable compound.
Ammonia (NH3): One nitrogen atom shares its electrons with three hydrogen atoms in ammonia. Each hydrogen atom shares one electron, and the nitrogen atom shares three, achieving stability for all involved atoms.
Ethanol (C2H5OH): Ethanol is a more complex example involving multiple types of atoms. Two carbon atoms, six hydrogen atoms, and one oxygen atom share electrons in a specific arrangement to form this compound.
Methane (CH4): Methane comprises one carbon atom and four hydrogen atoms. Each hydrogen atom shares one electron, and the carbon atom shares four, making it a stable compound.
Sulfur Hexafluoride (SF6): This compound involves one sulfur atom and six fluorine atoms. Each fluorine atom shares one electron, and the sulfur atom shares six, achieving stability for all atoms.
Water (H2O): One oxygen atom bonds with two hydrogen atoms in water. Each hydrogen atom shares one electron, and the oxygen atom shares two, making it a stable compound.
Acetic Acid (CH3COOH): Acetic acid is another complex example. It involves two carbon atoms, four hydrogen atoms, and two oxygen atoms sharing electrons in a specific arrangement to form this compound commonly found in vinegar.
Practical Applications of Covalent Compounds
Covalent compounds have a wide range of applications. In pharmaceuticals, many drugs, like aspirin, are covalent compounds designed to interact with biological systems. Likewise, in the food industry, compounds like sucrose serve as sweeteners. Additionally, environmental science also deals with covalent compounds like methane, which is a significant greenhouse gas. Household products like window cleaners often contain covalent compounds such as ammonia. Covalent compounds like silicon dioxide manufacture glass in technology, and polymers in plastics are long chains of covalent compounds. Lastly, personal care products, from toothpaste to shampoo, also contain these compounds as active or inactive ingredients.
Conclusion
In conclusion, making covalent compounds is about atoms sharing electrons to reach a stable state. You start by picking nonmetals such as carbon or oxygen and then focus on their valence electrons - these outermost electrons are vital in forming the bond. After identifying the elements and their valence electrons, you can visualize the bonding through Lewis's dot structures or molecular models. From everyday items to advanced tech, covalent compounds are essential; now, you should better understand how they come together!
Summarise with AI:












Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic