Chapters
Atoms are the building blocks of everything around us, from the air we breathe to the water we drink. But how do these atoms stick together to form molecules? The answer lies in Dot and Cross diagrams. These diagrams are a bit like mini roadmaps showing how atoms share or swap tiny particles called electrons to form molecules. This guide aims to simplify the concept of these diagrams, explain their real-world relevance, and help you create one yourself. Read on below to find out more!

What Are Dot and Cross Diagrams?
Put, Dot and Cross diagrams are simple sketches that show how atoms bond to form molecules. In these diagrams, dots and crosses represent electrons, tiny particles that orbit the centre of an atom. Essentially, dots represent electrons from one atom, while crosses represent electrons from another. These diagrams show how atoms reach a stable state, often similar to noble gases, which are elements that don't like to react with others.
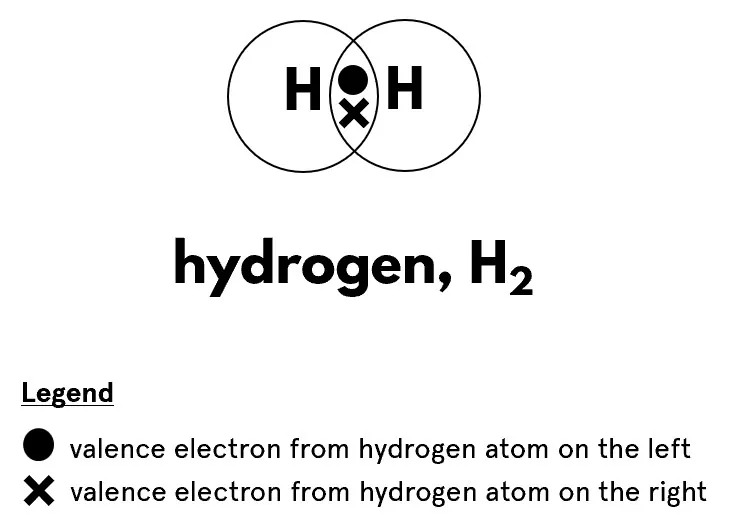
(Credit:chemnotcheem.com)
The Importance of Valence Electrons
Electrons are not all the same - in fact, the ones that play a significant role in bonding are called valence electrons. These are the electrons found in the outermost shell of an atom. Understanding the number of valence electrons an atom has is crucial for creating an accurate Dot and Cross diagram. Thankfully, you can easily find this information on the periodic table as each element is displayed along with its properties.
Step-by-Step: How to Make Dot and Cross Diagrams
Creating Dot and Cross diagrams is straightforward once you get the hang of it. Let's take a look at how to do it below.
Identify the Atoms Involved: First, determine which atoms you will work with within the molecule. Knowing the atoms will guide you in the subsequent steps.
Step Two: Next up, you'll want to determine the number of valence electrons for each atom. You can easily find this information on the periodic table. The valence electrons are crucial for bonding, so don't skip this step!
Step Three: Now, draw the symbols for each atom in a layout that mimics the actual molecular structure. This will give you a visual framework for the bonding that takes place.
Step Four: The "dot and cross" part comes in here. Surround the atom symbols with dots and crosses to signify their valence electrons. Make sure to place them in a way that shows which electrons belong to which atom.
Step Five: Draw lines or pairs of dots and crosses between the atoms. This will show how the atoms share or exchange electrons to form bonds.
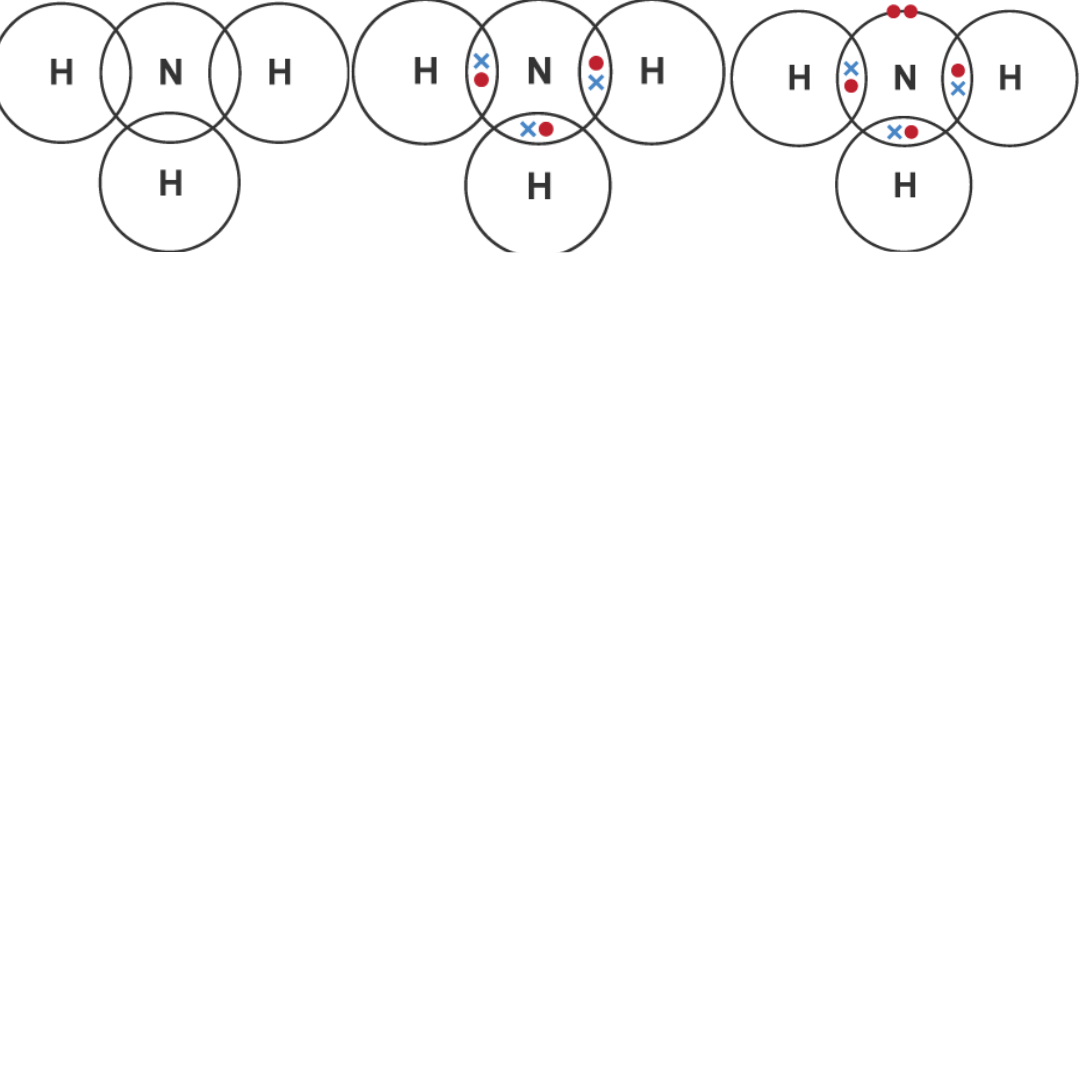
(Credit: BBC Bitesize)
Examples
Water (H2O): Starting with Oxygen, it has six valence electrons and takes the central position. Flanking it are two Hydrogen atoms, each with a single valence electron. To represent this, draw six dots around the Oxygen and a cross next to each Hydrogen. To show the sharing of electrons, pair a dot with a cross.
Carbon Dioxide (CO2): Moving on to Carbon Dioxide, Carbon is at the core with four valence electrons. It's joined by two Oxygen atoms, each with six valence electrons. To depict this, encircle Carbon with four dots and place six dots around each Oxygen. The double bond between Carbon and Oxygen is indicated by matching the dots.
Methane (CH4): Lastly, we have Methane. Carbon is back in the middle, this time with four valence electrons. Surrounding it are four Hydrogen atoms, each having one valence electron. Sketch four dots around Carbon and a cross beside each Hydrogen. Connect a dot and a cross to demonstrate that they're sharing electrons.
Different Types of Bonds
Additionally, Dot and Cross diagrams are versatile tools for illustrating different types of bonds. For instance, covalent bonds, common in organic compounds like water and Methane, involve the sharing of electrons between atoms. On the other hand, ionic bonds occur when one atom donates one or more electrons to another, as seen in table salt. Additionally, metallic bonds feature electrons shared among a network of atoms, a bonding style typical in metals such as copper and iron.
Real-World Uses
Dot and Cross diagrams have many practical applications, too. For example, understanding the molecular structure of different compounds in the pharmaceutical industry is crucial for developing exciting new medications. How do they do this? These diagrams offer a simplified yet detailed view of how atoms interact, which can be vital for creating effective drugs. In the field of material science, Dot and Cross diagrams also play a significant role in the development of new materials. Whether scientists are working on creating more robust plastics, more conductive metals, or more efficient semiconductors - understanding the atomic structure is a vital part of the job.
Additionally, regarding environmental science, these diagrams help researchers understand molecular interactions that occur in natural processes. This is particularly important when studying water purification methods or air quality control. By understanding how molecules interact at the atomic level, scientists can develop more effective ways to tackle environmental challenges.
Why Scientists Use Dot and Cross Diagrams
Dot and Cross diagrams find applications across a wide range of scientific fields, and their popularity can be attributed to several key advantages. Firstly, their simplicity - these diagrams provide a clear yet simplified representation of intricate molecular structures. Secondly, their predictive power offers valuable insights into how molecules behave and react, which can be crucial when planning experiments. Lastly, their educational value - these diagrams excel at breaking down complex ideas into more easily understandable terms, making them excellent tools for learning.

Advanced Concepts and Tips
Once you complete the basics, you can explore more advanced aspects of Dot and Cross diagrams. Some molecules can be represented by more than one valid Dot and Cross diagram, known as resonance structures. Additionally, these diagrams can help understand the polarity of molecules, which is crucial for predicting how they will interact with other substances. However, it's essential to be cautious. Always double-check your work, as a small mistake in counting electrons could lead to a disastrous error in the long run.
Conclusion
Dot and Cross diagrams are handy tools for understanding the complex world of chemical bonding. Not only do they help when it comes to visualizing how atoms achieve stable electron configurations, but they can also be applied in various scientific fields like pharmaceuticals, material science, and environmental studies. Moreover, the diagrams are simple yet complex and offer predictive insights into molecular behaviour and reactivity for scientists and students. Therefore, mastering the art of creating Dot and Cross diagrams is a valuable skill that could help your understanding of chemistry and its real-world applications. Whether you're a student, a budding scientist, or just someone curious about the world around you, these diagrams can help you better understand the fascinating world of atoms and molecules.
Summarise with AI:













Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic