Chapters
Have you ever wondered about the science behind everyday items like the salt on your fries or the aluminium on your bike? You're in the right place! This guide aims to demystify the world of ionic compounds, from their formation to their naming conventions and even the chemical reactions that bring them to life. So, read on below to find out more!

What Exactly Are Ionic Compounds?
Ionic compounds are more common than you think. For example, they're in the salt you sprinkle on your food, the minerals in the Earth's crust, and even the air you breathe. In layperson's terms, ionic compounds form when atoms interact. A metal atom, for example, loses electrons and transforms into a positively charged particle known as a cation. On the other hand, a nonmetal atom will gain those electrons and become a negatively charged particle - or an anion. These opposite charges attract each other, much like the north and south poles of magnets, forming an ionic compound.
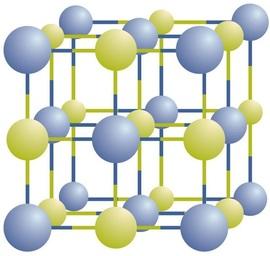
In a sodium chloride crystal, positive and negative ions are arranged in a 3D lattice and alternate their positions in the structure (Credit: Chemlibretexts)
The Golden Rule: Charge Balance
Understanding ionic compounds starts with a simple principle: the charges must balance. Imagine a seesaw on a playground - for it to be stable, both sides must be equal in weight. When it comes to ionic compounds, it's pretty much the same. For instance, magnesium carries a positive charge of two units in a compound made of magnesium and oxygen, while oxygen has a negative charge of two units. These charges balance each other out, resulting in a stable compound. Similarly, sodium has a single positive and negative charge in a compound involving sodium and chlorine - they're a perfect pair.
Writing Formulas: A Step-by-Step Guide
Writing the formula for an ionic compound can feel like solving a complex puzzle, but it doesn't have to feel impossible. Here's a step-by-step guide to make it easier.
Identify the Ions: The first step is to identify the ions that will combine. First and foremost, you'll need to know their charges, which you can usually find on the periodic table.
Write Down the Ions: Write down the names of the ions side by side, with the cation first and the anion second—for example, sodium (Na+) and chlorine (Cl-).
Balance the Charges: Ensure that the total positive charge equals the total negative charge. For example, one calcium ion will pair with two chloride ions to balance the charges.
Write the Name: Finally, write down the compound's name, using prefixes like "di-" or "tri-" if more than one of a particular ion is needed.
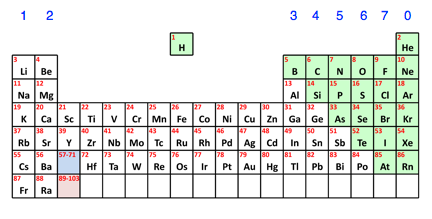
Periodic table (credit: chem guide)
Common Pitfalls to Avoid
There are a few pitfalls to watch out for when writing names for ionic compounds, too. Let's look at this below.
Not Balancing Charges: Always double-check that the total positive and negative charges balance out.
Incorrect Order: The cation should consistently be named first, followed by the anion.
Overcomplicating Polyatomic Ions: When dealing with ions made of multiple atoms, you'll need to use terms like "mono," "di," or "tri" to indicate the number of ions needed.
Transition Metals: Knowing the Difference
Transition metals are a unique case because they can have more than one type of charge. We use Roman numerals in the compound's name to clarify which charge we're referring to. For instance, in a compound made of iron and oxygen, where iron has a charge of three units, we call it "iron (III) oxide."
Practical Examples
Let's go through some examples to help you understand this even better. For example, when barium and chlorine combine, they form a compound known as barium chloride. One barium ion pairs with two chloride ions in this compound to balance the charges. Think of it like cooking - you mix the right ingredients in proportions to get a different type of meal.
How Scientists Create Ionic Compounds: The Laboratory Approach
In the lab, scientists use various unique techniques to create ionic compounds. For example, one of the most popular methods is using electricity. For instance, they will use electric currents to make table salt to change sodium and chlorine into charged particles or ions. These ions are then attracted to each other and form salt. Alongside this, another method they use is mixing two liquids that contain different ions. When mixed, these ions can form a new solid substance that settles at the bottom, and this new solid is another type of ionic compound. These lab methods are super important because they help us make essential things like medicines and better crops for farming.
Chemical Reactions Involving Ionic Compounds

Chemical reactions are where the real magic happens with ionic compounds. There are several types of reactions to know about:
Formation Reactions: A metal and a nonmetal combine to create an ionic compound. Like when sodium meets chlorine, and we get table salt.
Dissolution and Precipitation: Ionic compounds can dissolve in water, breaking into individual ions. Mix two different ion solutions, and you might get a new ionic compound that settles at the bottom.
Redox Reactions: These involve the transfer of electrons between atoms. One element loses electrons and oxidises, while another gains and gets reduced. These reactions are critical in things like batteries.
Acid-Base Reactions: Ionic compounds can also be part of acid-base reactions. For example, hydrochloric acid and sodium hydroxide neutralize each other to form water and table salt.
Conclusion
In summary, ionic compounds are formed through the intricate balance of cations and anions, each carrying its unique charge. Additionally, transition metals add another layer of complexity to the subject, as they can assume multiple charges. Scientifically crafted in labs, these compounds engage in various chemical reactions - from formation to dissolution. Learning about how ionic compounds are made is a fascinating topic that can appeal to both younger students and budding scientists. What's more, knowing how to correctly write their formulas and names and the chemical reactions they participate in can be helpful in everyday life. Whether it's the formation reactions that give us everyday substances like table salt or the more complex redox reactions that power our batteries, ionic compounds play a crucial role in nature and technology.
Summarise with AI:












Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic