Chapters

What is Water of Crystallisation?
Some salts contain water molecules trapped inside their crystal structure. These water molecules are not just sitting on the surface – they are chemically bonded into the lattice.
This water is called water of crystallisation.
Hydrated salt: a salt that contains water of crystallisation.
Anhydrous salt: a salt with no water of crystallisation (completely dry).
Degree of hydration: the number of water molecules chemically bound to each unit of the salt.
For example:
- The formula for hydrated copper(II) sulfate is CuSO₄·5H₂O.
- The dot (·) shows the water of crystallisation.
- It means for every 1 CuSO₄, there are 5 H₂O molecules in the crystal.
When hydrated copper(II) sulfate is heated, it loses its water of crystallisation and turns from blue crystals into a white powder of anhydrous copper(II) sulfate.
Why is Water of Crystallisation Important?
- It explains why some crystals appear coloured or have particular shapes.
- It allows us to calculate the formula of a salt by measuring how much water is lost on heating.
- It links to mole calculations, so it’s a common type of exam question.
Example: Calculating the % of Water in a Hydrated Salt
Let’s calculate the percentage of water in MgSO₄·7H₂O.
- Find the relative formula mass (Mr) of each part:
- MgSO₄ = 24 + 32 + (4 × 16) = 120
- 7H₂O = 7 × 18 = 126
- Add them together:
- Mr of MgSO₄·7H₂O = 120 + 126 = 246
- Calculate the percentage by mass of water:
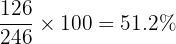
So, about 51% of the mass of magnesium sulfate crystals is just water!
Experimental Determination of Water of Crystallisation
A classic GCSE experiment involves heating a known mass of hydrated salt (e.g. CuSO₄·xH₂O) until no more water is lost. The method is outlined below:
- Weigh the hydrated salt.
- Heat strongly to drive off the water.
- Weigh the anhydrous salt.
- Calculate the mass of water lost.
- Convert masses into moles to find the ratio of salt : water.
Example (summary):
- 6.25 g of CuSO₄·xH₂O heated → 4.00 g anhydrous CuSO₄ left.
- Mass of water lost = 2.25 g.
- Moles of CuSO₄ = 4.00 ÷ 160 = 0.025
- Moles of H₂O = 2.25 ÷ 18 = 0.125
- Ratio = 1 : 5 → Formula is CuSO₄·5H₂O.
Practice Questions & Answers
The formula for hydrated copper(II) sulfate is CuSO₄·5H₂O.
Calculate the percentage by mass of water in these crystals.
(Relative atomic masses: Cu = 63.5, S = 32.0, O = 16.0, H = 1.0)
Mr(CuSO₄) = 63.5 + 32 + (4×16) = 159.5
Mr(5H₂O) = 5 × 18 = 90
Mr(total) = 159.5 + 90 = 249.5
% water = (90 ÷ 249.5) × 100 = 36.1% (3 s.f.)
Hydrated cobalt(II) chloride has formula CoCl₂·xH₂O.
On heating, 21.7% of the mass was water. Find x.
(Relative atomic masses: Co = 59.0, Cl = 35.5, O = 16.0, H = 1.0)
Assume 100 g hydrated salt → water = 21.7 g, CoCl₂ = 78.3 g
Mr(CoCl₂) = 59.0 + (2×35.5) = 130
n(CoCl₂) = 78.3 ÷ 130 = 0.602
n(H₂O) = 21.7 ÷ 18 = 1.206
Ratio CoCl₂ : H₂O = 0.602 : 1.206 ≈ 1 : 2
So x = 2 → CoCl₂·2H₂O.
A sample of hydrated iron(II) sulfate FeSO₄·xH₂O weighed 10.20 g.
After heating, 5.57 g of anhydrous salt remained. Find x.
(Relative atomic masses: Fe = 55.8, S = 32.0, O = 16.0, H = 1.0)
Water lost = 10.20 − 5.57 = 4.63 g
Mr(FeSO₄) = 55.8 + 32 + (4×16) = 151.8
n(FeSO₄) = 5.57 ÷ 151.8 = 0.0367
n(H₂O) = 4.63 ÷ 18 = 0.257
Ratio FeSO₄ : H₂O = 0.0367 : 0.257 ≈ 1 : 7
So x = 7 → FeSO₄·7H₂O.
Sodium sulfate crystals have formula Na₂SO₄·xH₂O.
A 3.578 g sample was heated to 1.578 g. Find x.
(Relative atomic masses: Na = 23.0, S = 32.0, O = 16.0, H = 1.0)
Water lost = 3.578 − 1.578 = 2.000 g
Mr(Na₂SO₄) = (2×23) + 32 + (4×16) = 142
n(Na₂SO₄) = 1.578 ÷ 142 = 0.0111
n(H₂O) = 2.000 ÷ 18 = 0.111
Ratio Na₂SO₄ : H₂O = 0.0111 : 0.111 ≈ 1 : 10
So x = 10 → Na₂SO₄·10H₂O (Glauber’s salt).
GCSE/iGCSE Exam tips!
- Always find the mass of water lost first.
- Convert all masses to moles.
- Divide by the smallest number of moles for a whole-number ratio.
- If you’re slightly off a whole number (e.g. 1.98, 2.02), round sensibly based on experimental error.
Summarise with AI:












Lithium is used in place of Fluorine
Hi Madan. You’re right to point that out – thanks very much for your comment!
amazing side
It’s very educative,I learnt alot thanks ❤️
Was very educative and help. Gave me a broader understanding of the topic