Chapters
Conduction, convection, and radiation are fundamental concepts in thermodynamics. They play a significant role in our daily lives, from keeping our food warm to cooling our computers. In this article, we will explore these concepts in depth, providing real-life examples to help you understand the differences between them. Additionally, we will discuss how to calculate the energy involved in each process and the various factors that affect them.

Transfer of Heat
The transfer of heat energy can occur through different methods such as conduction, convection, or radiation. The energy always flows from a region of high temperature to a region of low temperature.
Conduction
Materials such as metals are known to be good conductors of heat energy, while non-metals and gases are usually poor conductors, also known as insulators.
Conduction in Solids
In solids, the atoms are fixed in place but can vibrate, and heat energy is transferred by the vibrations of the atoms. This process is relatively slow.
Conduction in Metals
In contrast, in metals, some of the electrons can move freely and absorb heat energy, and then move quickly and crash into positively charged metal ions, which makes the process much faster than in non-metals.
Hence, we can summarize the process of conduction like this:
- In non-metals or insulators, the process of conduction occurs only by the transfer of vibrations between atoms.
- However, in good conductors like metals, conduction is caused by both the transfer of vibrations between atoms and collisions between free electrons and metal ions.
- The conduction by collisions between free electrons is significantly faster than conduction by transfer of vibrations between atoms.
- Due to the presence of free electrons, metals are excellent conductors of heat and electricity.
Calculating the Rate of Conduction
We can calculate the rate of conduction by using the equation below:
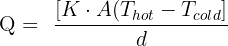
Here:
Q = Heat transfer per unit time
K = Thermal conductivity of the body
A = Area of heat transfer
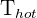 = Temperature of hot region
= Temperature of hot region
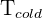 = Temperature of cold region
= Temperature of cold region
d = thickness of the body
Convection
Heat can be transferred from one place to another in liquids or gases by convection.
Here are some unique points to consider:
- Convection occurs when particles with higher thermal energy in a fluid move to a place with lower thermal energy.
- Liquids and gases are called fluids because their particles can move around freely and change places with each other.
Convection in Liquids
- In liquids, convection can be seen by adding a small amount of dye or crystals to the liquid and gently heating it. The heated particles move faster and rise to the top, while cooler particles sink to the bottom. This creates a circular motion that can be observed.
- In gases, convection can be seen in many natural phenomena such as the circulation of air in the atmosphere, the formation of thunderstorms, and the movement of ocean currents.
- The transfer of heat through the glass wall of the beaker occurs initially by conduction.
- When a region of water is heated by the Bunsen flame, it expands, becomes less dense, and moves up.
- Cooler, denser water around it replaces it, which also heats up, expands, and moves up.
- This cycle goes on, generating a convection current and transferring heat through the liquid.
- Convection currents can be observed in lava lamps as well, where the wax inside the lamp warms up, rises up due to being less dense than the liquid, and creates a cycle.
Convection in a Gas
- Convection currents are responsible for heat transfer through the air.
- It occurs when the air close to the radiator is heated, which causes it to expand and become less dense, and as a result, it rises. Cooler and denser air surrounding it moves in to replace the heated air.
- This cooler air gets heated in turn, expands, becomes less dense and rises, continuing the process of convection currents, transferring heat through the air and into the room.
- Convection currents also explain why upstairs in a house is often hotter than downstairs.
- On a larger scale, convection currents also contribute to the formation of many ocean currents, as well as being responsible for most of the winds we experience.
- Furthermore, convection currents enable hot air balloons to rise and move in the sky.
Calculating the Rate of Convection
Use the equation below to calculate the rate of convection:
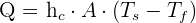
Here:
Q = Heat transfer per unit time
A = Area of heat transfer
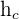 = coefficient of convective heat transfer
= coefficient of convective heat transfer
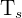 = surface temperature
= surface temperature
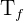 = fluid temperature
= fluid temperature
Radiation
- Infrared radiation is a form of energy that can transfer heat without requiring particles to vibrate or move.
- It is a type of electromagnetic radiation that travels through the air and can be absorbed by objects.
- When an object absorbs infrared radiation, its temperature increases and it becomes heated.
- This type of heat transfer is commonly used in cooking, such as in microwave ovens, where food is heated by absorbing microwave radiation.
- It is also used in heating systems, such as outdoor heaters and some types of industrial processes.
Calculating Radiation
Radiation can be calculated Stefan-Boltzmann law using the following eqi=uation:
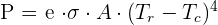
Here:
P = net power of radiation
A = Radiation area
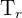 = Temperature of the radiator
= Temperature of the radiator
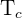 = Temperature of the surroundings
= Temperature of the surroundings
e = emissivity
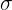 = Stefan's constant which is equal to
= Stefan's constant which is equal to 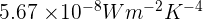
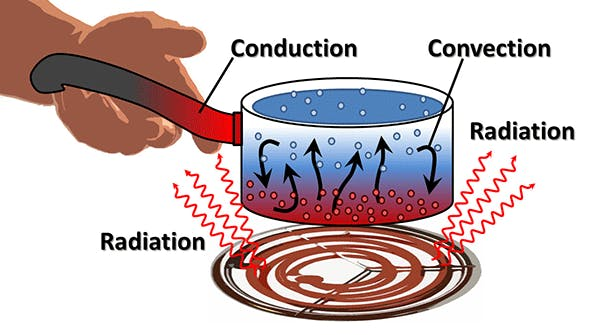
Examples of Conduction, Convection, and Radiation From Everyday Life
Here are some examples of conduction, convection, and radiation from everyday life:
Conduction:
- When you touch a metal spoon that has been left in hot tea, the spoon conducts heat from the tea to your hand.
- On a cold day, heat is conducted from your body to the ground when you sit on a metal bench.
- When you cook food in a frying pan, heat is conducted from the pan to the food.
Convection:
- On a hot day, warm air rises and cooler air sinks, creating a convection current in the atmosphere.
- When you turn on a heater in a room, the warm air rises and cooler air is drawn towards the heater, creating a convection current in the room.
- When you boil water in a pot, the water at the bottom of the pot is heated, expands, and rises to the top, creating a convection current.
Radiation:
- When you stand in front of a fire, you feel warm because of the infrared radiation emitted by the fire.
- On a sunny day, you feel warm because of the infrared radiation emitted by the sun.
- When you heat food in a microwave, the food is heated by electromagnetic waves, including infrared radiation.
Factors Affecting Conduction, Convection, and Radiation
Many factors affect conduction, convection, and radiation which include:
- Temperature difference: The greater the temperature difference between two objects, the faster the transfer of heat energy between them.
- Surface area: The larger the surface area of an object, the greater the amount of heat energy that can be transferred through it.
- Material properties: The thermal conductivity of a material affects how easily it can transfer heat energy through conduction. Materials that are good conductors, such as metals, transfer heat more quickly than poor conductors, such as insulators.
- Density: In convection, the density of the fluid affects the speed at which it can transfer heat energy. A less dense fluid will rise more quickly than a denser fluid.
- Distance: The distance between two objects affects the amount of heat energy that can be transferred through radiation. The closer the objects, the more heat energy that can be transferred.
- Surface properties: The surface properties of an object, such as color and texture, can affect how much heat energy it absorbs or reflects. Darker and rougher surfaces absorb more heat energy than lighter and smoother surfaces.
- Environmental factors: Environmental factors such as humidity and wind speed can affect how quickly heat energy is transferred through convection and radiation. High humidity can slow down convection, while high wind speeds can increase the rate of heat loss through convection and radiation.
Summarise with AI:













You are the best,, coz you have gotten content about the topics
Hello ! Glad to hear that you’ve found the content useful!