Chapters
Are you ready to uncover the mystery of specific heat capacity? In this article, we'll explore what it is, how to calculate it, and its units of measurement. We'll also take a look at some real-life examples where specific heat capacity plays a vital role.

What is Specific Heat Capacity?
Modifications in the temperature or state of matter of a substance can be attributed to changes in its internal energy. The amount of energy necessary for these changes is determined by the material's specific heat capacity and latent heat. The specific heat capacity of a material can be defined as:
"The heat capacity of a material refers to the amount of energy required to raise its temperature by a certain amount"
Different materials possess varying heat capacities due to differences in their chemical composition and molecular structure.
Units of Specific Heat Capacity
The unit of specific heat capacity is joules per kilogram per degree Celsius (J/kg°C) or joules per kilogram per Kelvin (J/kgK).
Factors Affecting Specific Heat Capacity of an Object
It is observed that the change in temperature of a system depends on various factors, such as:
- the mass of the material
- and substance of the material
- the amount of energy added to the system.
Understanding these factors is crucial in predicting and explaining temperature changes in different systems.
Specific Heat Capacity of Water Vs Lead
The specific heat capacity of a substance is a measure of the amount of heat energy required to raise the temperature of a given mass of the substance by one degree Celsius.
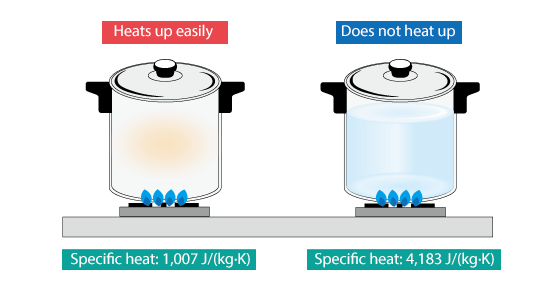
Lead has a low specific heat capacity of 128 J/kg/°C, which means that it requires only a small amount of energy to raise its temperature. This makes lead a good conductor of heat, as it can quickly transfer energy from one place to another. When a one kilogram block of lead absorbs heat, the particles within it experience an increase in energy. As lead is a solid, the particles only vibrate, but their vibration rate speeds up when heated. The close proximity of the particles in a solid means that energy is efficiently transferred as particles collide and distribute energy among each other.
Consequently, the energy spreads rapidly throughout the lead block, causing the temperature to rise quickly. Notably, it requires less energy to increase the temperature of a one kg block of lead by 1°C than it does for one kg of water. In the case of water, it has a specific heat capacity of 4,200 J/kg°C, which means that it requires 4,200 joules of energy to increase the temperature of one kilogram of water by one degree Celsius.
This property of water is of significant importance in many fields, including thermodynamics, engineering, and meteorology. For instance, the high specific heat capacity of water allows it to absorb and store a large amount of heat energy from the sun, which in turn helps to regulate the Earth's temperature and climate. It also plays a crucial role in the cooling systems of many industrial processes, as well as in heating and cooling systems used in homes and buildings.
Calculating Specific Heat Capacity
Specific heat capacity of a substance can be measured using the equation below:
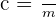
Here:
c = specific heat capacity
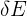 = change in thermal energy
= change in thermal energy
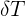 = change in temperature
= change in temperature
Example
A 2 kg block of aluminum is heated from 25°C to 75°C using 10,000 J of energy. What is the specific heat capacity of aluminum?
Solution
First, we need to calculate the change in temperature of the aluminum block:
ΔT = T₂ - T₁
ΔT = 75°C - 25°C
ΔT = 50°C
Next, we can use the formula for specific heat capacity:
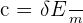
where:
- c is the specific heat capacity
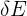 is the amount of energy transferred
is the amount of energy transferred- m is the mass of the material
- ΔT is the change in temperature
Plugging in the given values, we get:
c = 10,000 J / (2 kg * 50°C)
c = 100 J/kg°C
Therefore, the specific heat capacity of aluminum is 100 J/kg°C.
Calculating Thermal Energy Changes
Use the following equation to calculate thermal energy changes:
Q = mcΔT
where:
- Q = thermal energy change (in joules)
- m = mass of the substance (in kilograms)
- c = specific heat capacity of the substance (in joules per kilogram per degree Celsius)
- ΔT = change in temperature (in degrees Celsius)
Example
Calculate the thermal energy change when 500 grams of water (c = 4,200 J/kg°C) is heated from 20°C to 100°C.
Solution
First, we need to convert the mass to kilograms:
m = 500 g / 1000 g/kg = 0.5 kg
Next, we can plug in the values into the formula:
Q = (0.5 kg) x (4,200 J/kg°C) x (100°C - 20°C)
Q = 0.5 x 4,200 x 80 = 168,000 joules
Therefore, it takes 168,000 joules of thermal energy to heat 500 grams of water from 20°C to 100°C.
Examples of Specific Heat Capacity From Everyday Life
Here are some examples of specific heat capacity from everyday life:
- Use of hot water in a bottle: When hot water is poured into a rubber or plastic container and sealed, it remains hot for a long period of time. This is because water has a relatively high specific heat capacity (4,200 J/kg°C), which means it can absorb and store a large amount of heat energy before its temperature rises significantly. This makes water an effective medium for storing and transferring heat energy in various applications, including heating systems, cooking, and cooling.
- Cooking: Specific heat capacity is important in cooking as it determines how quickly food will heat up or cool down. For example, when cooking a steak, a cast iron skillet is often used because it has a high specific heat capacity, meaning it can absorb and retain heat well. This allows for a quick and even cooking of the steak.
- Home insulation: Specific heat capacity is also important in home insulation. Materials with a high specific heat capacity, such as concrete, can absorb and store heat well. This can be useful in regions with hot summers and cool winters, as it can help regulate the temperature inside a building by absorbing heat during the day and releasing it at night.
- Cooling systems: The specific heat capacity of a coolant, such as water or antifreeze, is important in the design of cooling systems for cars, computers, and other machinery. These systems rely on the absorption of heat by the coolant to prevent the machinery from overheating.
- Building materials: The specific heat capacity of building materials, such as concrete and brick, can affect how quickly a building heats up or cools down. Materials with high specific heat capacity, like brick, can help to regulate the temperature inside a building by absorbing and releasing thermal energy slowly.
Summarise with AI:













You are the best,, coz you have gotten content about the topics
Hello ! Glad to hear that you’ve found the content useful!