Chapters
From the fuel in your car to the food you eat, chemical energy is all around us. But what exactly is chemical energy, and how does it work? In this article, we'll explore the basics of chemical energy, including its sources, how it's calculated, and the factors that can affect its strength. Join us on a journey into the fascinating world of chemical reactions and discover the power of this essential energy source.

Chemical Energy Defined
We can define chemical energy as:
"The energy that is stored in the chemical bonds between atoms and molecules within a substance is known as chemical energy"
This energy is potential energy that can be released when a chemical reaction takes place, such as when fuel is burned. During a chemical reaction, the bonds between atoms and molecules are broken, and the stored chemical energy is released in the form of heat and/or light. This energy can be harnessed to perform work, such as powering a car or generating electricity.
Sources of Chemical Energy
Some of the common sources of chemical energy are:
- Fossil fuels: They are a major source of chemical energy that can be burned to release stored energy.
- Biofuels: They are a renewable source of chemical energy that can be made from plant materials.
- Food: It is a source of chemical energy that is converted into usable energy through cellular respiration.
- Batteries: They store chemical energy that can be converted into electrical energy.
- Chemical reactions: For example, the reaction between acids and alkalis, can also release stored chemical energy.
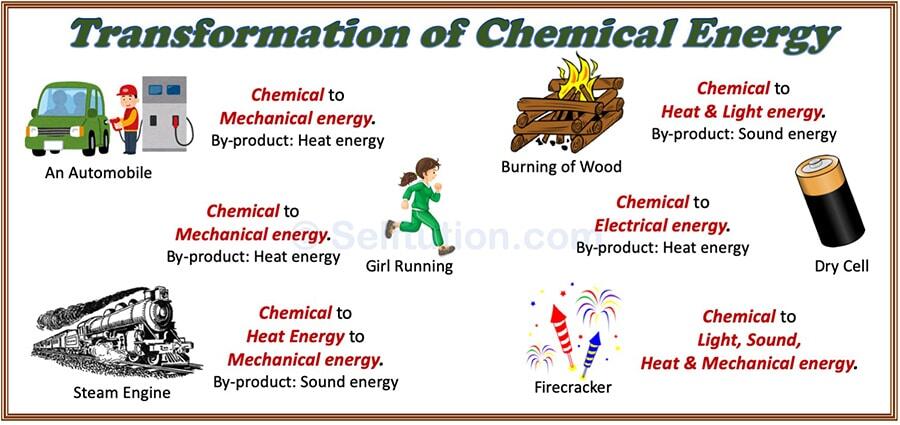
How is Chemical Energy Converted in Other Forms of Energy?
Chemical energy to thermal energy
When chemical reactions occur, such as burning a fuel, the chemical energy stored in the bonds between atoms and molecules is converted into thermal energy. This is the heat released by the reaction, and it can be used for a variety of purposes, such as heating a room or cooking food.
Chemical energy to electrical energy
Chemical energy can also be converted into electrical energy through processes such as batteries or fuel cells. In these processes, the chemical energy stored in the fuel is converted into electrical energy through a chemical reaction. This electrical energy can then be used to power devices such as electric motors, light bulbs, and electronic devices.
Chemical energy to kinetic energy
In addition, chemical energy can be converted into kinetic energy, which is the energy of motion. This can occur when chemical energy is used to power engines or motors, such as in cars, airplanes, and rockets. The chemical energy in the fuel is converted into kinetic energy, which propels the vehicle forward.
Loss of Chemical Energy During Conversion
It's important to note that the conversion of chemical energy into other forms of energy is not always 100% efficient. Some of the energy may be lost in the form of waste heat or sound. However, the conservation of energy principle dictates that energy cannot be created or destroyed, only transformed from one form to another.
Understanding how chemical energy can be transferred and transformed into other forms of energy is an important part of understanding the role of energy in our lives and in the world around us.
Effects of Chemical Energy on Environment
Chemical energy is often used to power vehicles, generate electricity, and heat buildings, and these processes can have negative impacts on the environment.
Pollutants released as a result of fossil fuel burning
When fossil fuels such as coal, oil, and natural gas are burned, they release pollutants into the air, including carbon dioxide, nitrogen oxides, and sulfur dioxide. These pollutants can contribute to smog, acid rain, and respiratory problems in humans and other animals. In addition, the release of carbon dioxide and other greenhouse gases contributes to climate change, which has a range of negative impacts on ecosystems, human health, and the economy.
Effects of biofuels
Biofuels, which are made from plant matter or animal waste, can also have environmental impacts. While they are renewable and produce fewer emissions than fossil fuels, the production and transportation of biofuels can still contribute to greenhouse gas emissions, land use changes, and water pollution.
How to Calculate Chemical Energy?
To calculate the energy released from a substance use the following formula:
Chemical energy = mass of substance x specific heat capacity x change in temperature
This formula can be used to determine the amount of energy released when a substance undergoes a chemical reaction, such as combustion.
Here:
- The mass of the substance refers to the amount of material involved in the reaction, typically measured in grams or kilograms.
- The specific heat capacity is a property of the substance that describes how much energy is required to raise its temperature by a certain amount. This is typically measured in joules per kilogram per degree Celsius (J/kg°C).
- The change in temperature refers to the difference in temperature between the reactants and products of the chemical reaction. This can be measured using a thermometer and is typically expressed in degrees Celsius (°C).
By multiplying these three values together using the formula, students can calculate the chemical energy released by the reaction. It's important to note that the specific heat capacity of a substance can vary depending on factors such as temperature and pressure, so accurate measurements are important for reliable calculations.
Factors Affecting Chemical Energy
Some of the factors that affect chemical energy are:
- Chemical composition: Different substances have different chemical compositions, which affects the amount of chemical energy that can be released or stored. For example, hydrocarbons contain more chemical energy per unit mass than carbohydrates.
- Bond strength: The strength of chemical bonds affects the amount of energy required to break those bonds and release the stored chemical energy. Stronger bonds contain more stored energy than weaker bonds.
- Reactivity: The reactivity of a substance affects its ability to undergo chemical reactions and release energy. Highly reactive substances have a greater potential for releasing chemical energy than less reactive substances.
- Combustion conditions: The conditions under which combustion occurs affect the amount of chemical energy released. For example, complete combustion releases more energy than incomplete combustion.
- Conservation of energy: The law of conservation of energy applies to chemical reactions, meaning that the total amount of energy before and after a reaction must be equal.
Summarise with AI:












You are the best,, coz you have gotten content about the topics
Hello ! Glad to hear that you’ve found the content useful!