Chapters
Get ready to heat things up with a deep dive into the world of thermal energy! From the flicker of a flame to the warmth of the sun, thermal energy is all around us, powering everything from our homes to our vehicles. In this article, we'll explore the basics of this powerful force, including its sources, how it's calculated, and the factors that can affect its strength.

Thermal Energy Defined
We can define thermal energy as:
The energy that is associated with the temperature of a substance is known as thermal energy
The temperature of an object is determined by the amount of energy stored in its thermal store. Thermal energy is the sum of the kinetic energy of the particles (atoms or molecules) that make up a substance. The faster the particles move, the higher the thermal energy.
Thermal energy can be transferred to and from a system or an object. Thermal energy can be transferred between objects through conduction, convection, and radiation. It can also be converted into other forms of energy, such as mechanical energy or electrical energy. The unit of measurement for thermal energy is joules (J).
Sources of Thermal Energy
Some of the main sources of thermal energy include:
- Sun: The sun is a major source of thermal energy on Earth. The energy from the sun is transferred to Earth through radiation, which heats up the atmosphere, oceans, and land.
- Combustion: Combustion of fuels like coal, oil, natural gas, and wood releases thermal energy.
- Friction: Friction between two surfaces generates heat, which is a form of thermal energy.
- Electrical appliances: Electrical appliances like heaters, toasters, and ovens convert electrical energy into thermal energy.
- Chemical reactions: Chemical reactions can either produce or consume thermal energy. For example, exothermic reactions release thermal energy, while endothermic reactions absorb thermal energy.
- Geothermal energy: Geothermal energy is thermal energy stored beneath the Earth's surface, generated by the decay of radioactive materials in the Earth's core and mantle.
- Human metabolism: The human body produces thermal energy as a by-product of metabolism, maintaining a constant body temperature.
How to Calculate Thermal Energy?
To raise the temperature of a given mass of a substance by a given amount, a certain amount of energy is required. This amount can be calculated using the equation:
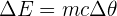
Here:
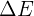 = change in thermal energy, measured in joules (J)
= change in thermal energy, measured in joules (J)- m = mass of the substance, measured in kilograms (kg)
- c = specific heat capacity of the substance, measured in joules per kilogram per degree Celsius (J/kg °C)
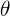 = change in temperature, measured in degrees Celsius (°C)
= change in temperature, measured in degrees Celsius (°C)
Specific heat capacity of a substance
The specific heat capacity of a substance is defined as:
The amount of energy required to raise the temperature of 1 kg of the substance by 1 °C
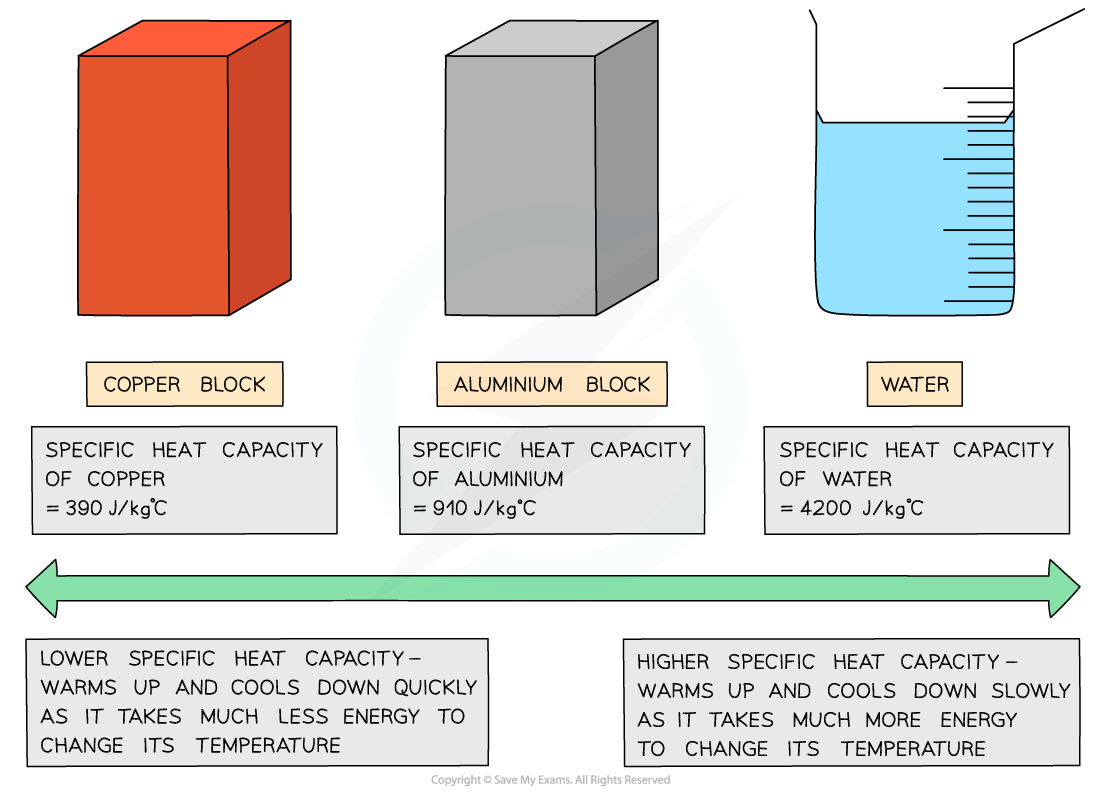
Different substances have different specific heat capacities.
- If a substance has a low specific heat capacity, it heats up and cools down quickly, meaning it takes less energy to change its temperature.
- On the other hand, if a substance has a high specific heat capacity, it heats up and cools down slowly, meaning it takes more energy to change its temperature.
Low vs high specific heat capacity
- The specific heat capacity of solids and liquids is commonly used to characterize the amount of thermal energy required to change their temperature.
- It also plays an important role in choosing suitable materials for various applications, such as selecting the most efficient material for kitchen appliances.
- Materials with low specific heat capacity, like copper and lead, are often excellent conductors of heat and electricity.
- Conversely, water has a high specific heat capacity, allowing it to retain heat for an extended period of time, making it ideal for heating homes through radiators.
Example 1
A 500 g piece of iron is heated from 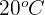 to
to 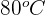 . What is the change in thermal energy of the iron?
. What is the change in thermal energy of the iron?
Solution
Step 1: Calculate change in temperature
First, we need to calculate the change in temperature:
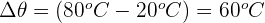
Step 2: Determine specific heat capacity of iron
Next, we need to find the specific heat capacity of iron, which is 0.45 J/g°C.
Step 3: Write down the equation
Now we can use the equation:
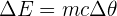
where 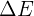 is the change in thermal energy, m is the mass of the object, c is the specific heat capacity, and
is the change in thermal energy, m is the mass of the object, c is the specific heat capacity, and 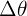 is the change in temperature.
is the change in temperature.
Step 4: Substitute values in the equation
Substituting the values, we get:
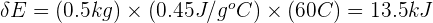
Therefore, the change in thermal energy of the iron is 13.5 kJ.
Example 2
A 2 kg block of copper initially at 20°C is heated until its temperature reaches 80°C. Calculate the thermal energy transferred to the copper block during the process, given that the specific heat capacity of copper is 386 J/kg °C.
Solution
Step 1: Write down the known values
m = 2 kg
c = 386 J/kg °C
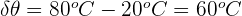
Step 2: Write down the equation
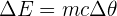
Step 3: Substitute values in the equation
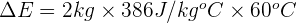
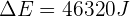
Therefore, the thermal energy transferred to the copper block during the process is 46,320 J.
Factors Affecting Thermal Energy of an Object or System
Many factors affect the thermal energy of an object or a system.
- Mass: The greater is the mass of an object or system, the more thermal energy it will contain.
- Temperature difference: The larger the difference in temperature between two objects or systems, the more thermal energy will be transferred from the hotter object to the colder object.
- Specific heat capacity: The specific heat capacity of a substance determines how much thermal energy is required to raise its temperature by a given amount. Substances with higher specific heat capacities require more energy to raise their temperature than substances with lower specific heat capacities.
- Thermal conductivity: The thermal conductivity of a substance determines how quickly it can transfer thermal energy. Substances with higher thermal conductivity can transfer thermal energy more quickly than substances with lower thermal conductivity.
- Insulation: The effectiveness of insulation can also affect the amount of thermal energy present in a system. Good insulation can prevent the transfer of thermal energy, while poor insulation can allow thermal energy to escape.
- Phase changes: During a phase change, such as melting or boiling, the temperature of a substance remains constant even though thermal energy is being added or removed. This is because the added or removed energy is used to change the state of the substance rather than to increase its temperature.
Summarise with AI:












You are the best,, coz you have gotten content about the topics
Hello ! Glad to hear that you’ve found the content useful!