Chapters
Are you ready to dive into the fascinating world of changes of state? In this article, we'll unravel the mysteries of how substances change from one state to another. From the factors that affect changes of state, such as temperature and pressure, to calculating the energy involved, we've got you covered. And to make it more relatable, we'll also share some real-life examples of changes of state that you encounter in your daily routine. Get ready to expand your knowledge and gain a deeper understanding of the physical properties of matter.

Three States of Matter
There are three states of matter: solid, liquid, and gas.
Solids
Solids are made up of particles that are arranged in a pattern, forming a shape called a lattice. The particles in the lattice are connected by bonds and vibrate, with their motion depending on the temperature. The higher the temperature, the more energetic the particles become, resulting in an increased amplitude of vibration within the lattice. This vibration causes the solid to expand, a phenomenon known as thermal expansion.
Liquids
As a substance undergoes a phase change from solid to liquid, the structure of the material begins to disintegrate. The particles within the liquid no longer maintain a fixed arrangement and are now able to move more freely. Their movement is entirely random, with no set direction of motion.
Although bonds between particles still exist within the liquid, they are now bonded together into smaller, independent groups that can move freely within the liquid. This state of matter allows the particles to flow, conform to the shape of their container, and enables the material to take on a fluid-like property. The unique characteristic of liquids, such as viscosity and surface tension, is attributed to the nature of these inter-particle interactions.
Gases
Gas particles possess an increased level of kinetic energy compared to those found in solids or liquids. As a result of their heightened energy, the bonds between particles are typically broken, except in some gases where the particles may exist as molecules comprising two or more atoms bonded together, such as oxygen (O2).
The particles in a gas move at an accelerated pace and in entirely random directions. This random motion of gas particles leads to their tendency to fill any space available, uniformly spreading throughout the container they occupy. Gases are unique in their ability to exert pressure uniformly on all surfaces of their container, a characteristic that is exploited in various applications such as gas storage and transportation.

Changes of State of Matter
A change of state is defined as:
"A change in state, also known as a phase transition, is a physical process that involves the transformation of matter from one state to another, such as from a solid to a liquid or from a liquid to a gas"
Particles change the states of matter (solid, liquid, and gas) when they absorb heat energy. The particles will continue to absorb heat energy until they reach a point where they undergo a state change, at which point their temperature will stop rising. The increase in kinetic energy of the particles causes the changes of state from solids to liquids and gasses.
The amount of energy required to raise the temperature of a substance is called its specific heat capacity and it is unique to each material. Knowing the specific heat capacity of a material is essential for understanding how much heat energy is needed to change its temperature.
Kinetic Theory
When a substance is subjected to heat, its internal energy, also referred to as thermal energy, increases, leading to an increase in the movement of its constituent particles. As the thermal energy increases, so does the kinetic energy of the particles. When a sufficient amount of thermal energy has been absorbed by the particles, they gain enough energy to initiate a change in state.
During this phase transition, the energy being absorbed is utilized to break the intermolecular bonds between the particles, instead of increasing the temperature of the substance. This results in a plateau-like temperature, where the temperature of the substance remains constant until the phase transition is complete. As the bonds between the particles continue to weaken, the particles become more separated and move around more freely, resulting in a change in the physical properties of the substance, such as its volume and shape.
Calculating the Energy Involved in Changes of State
The change in energy involved in changes of state can be calculated using the equation below:
Change in Energy = mass x specific heat capacity x change in temperature
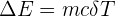
Example
How much energy is required to heat up 500 grams of iron from 25°C to 500°C?
Solution
The specific heat capacity of iron is 0.45 J/g°C. To solve this problem, we can use the following formula:
Q = m x c x ΔT
Where Q is the energy required, m is the mass of the substance (in grams), c is the specific heat capacity of the substance, and ΔT is the change in temperature.
In this case:
m = 500 g
c = 0.45 J/g°C
ΔT = (500°C - 25°C) = 475°C
Plugging these values into the formula, we get:
Q = 500 g x 0.45 J/g°C x 475°C
Q = 106,875 J or 106.9 kJ (rounded to one decimal place)
Therefore, it requires 106.9 kilojoules (kJ) of energy to heat up 500 grams of iron from 25°C to 500°C.
Factors Affecting Change of State
Many factors affect change of state of matter, some of which are discussed below:
- Temperature: Increasing the temperature of a substance can cause it to change from a solid to a liquid or from a liquid to a gas by increasing the kinetic energy of the particles, causing them to move faster and overcome the intermolecular forces holding them in place.
- Pressure: Increasing the pressure of a gas can cause it to change to a liquid or solid state by reducing the volume of the gas, and thus reducing the distance between the particles, which increases the intermolecular forces between them.
- Type of substance: Different substances have different intermolecular forces, and therefore require different amounts of energy to change state. For example, water requires more energy to change from a liquid to a gas than alcohol does.
- Particle size and shape: The size and shape of particles in a substance can affect its physical properties, including its melting and boiling points. For example, smaller particles may have a lower melting or boiling point than larger particles due to a higher surface area-to-volume ratio, which can cause them to overcome intermolecular forces more easily.
Examples of Change of State From Everyday Life
Here are some unique examples of changes of state from everyday life:
- Ice melting: When you take ice cubes out of the freezer and leave them at room temperature, they will melt and turn into water. This is an example of a change of state from a solid to a liquid, caused by an increase in temperature.
- Boiling water: When you heat water on a stove or in a kettle, it will eventually reach its boiling point and turn into steam. This is a change of state from a liquid to a gas, caused by an increase in temperature.
- Condensation on a cold surface: When warm, moist air comes into contact with a cold surface, such as a window on a cold day, the water vapor in the air will condense into droplets of water. This is a change of state from a gas to a liquid, caused by a decrease in temperature.
- Freezing water: When you put water in the freezer, it will eventually reach its freezing point and turn into ice. This is a change of state from a liquid to a solid, caused by a decrease in temperature.
- Evaporation of water: When you leave a puddle of water outside on a hot day, it will eventually dry up as the water molecules escape into the air as water vapor. This is a change of state from a liquid to a gas, caused by an increase in temperature.
Summarise with AI:













You are the best,, coz you have gotten content about the topics
Hello ! Glad to hear that you’ve found the content useful!