Chapters
In this article, we will discuss the general structure of amino acids and the creation and breakage of the peptide bond. Besides this, we will also discuss the terms primary, secondary, tertiary, and quaternary structure of proteins. But before discussing the structure of amino acids, first, let us recall what are proteins.

What are Proteins?
Proteins refer to the macromolecules and polymers that are composed of monomers known as amino acids. The shape and the function of amino acids are attributed to the number, type, and sequence of amino acids within them. Proteins are vital for the human body as they form enzymes, hormones, cell membrane proteins, transport proteins, immunoproteins, contractile proteins, and structural proteins.
Now, let us discuss amino acids.
What are Amino acids?
Amino acids refer to the monomers of proteins. 20 amino acids are present in proteins that are commonly present in all living organisms.
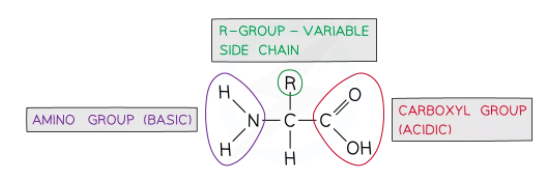
Generally, an amino acid has the following structure:
- A carbon atom in the center is bonded to:
- An amine group (
 )
) - A hydrogen atom
- A carboxylic group (COOH)
- An R group (The differences between each amino acid and its properties is determined by an R group. For instance, it determines whether an amino acid is acidic or basic, polar or non-polar).
The generalized structure of amino acid is shown in the diagram below:
In the next section of the article, we will discuss how a peptide bond is created and broken.
Peptide Bond
The Creation of a Peptide Bond
A peptide bond is created when the following two conditions are met:
- A loss of hydroxyl group (-OH) from a carboxylic group of single amino acid occurs
- A loss of hydrogen atom from an amine group of another amino acid
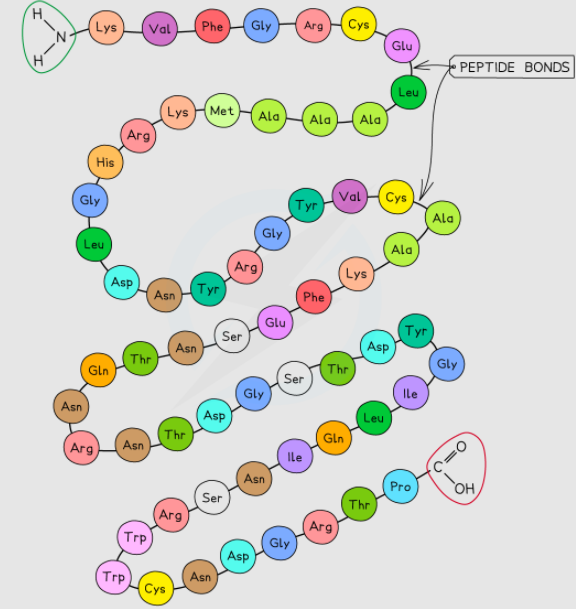
The carbon atom left (with the double-bonded oxygen) from the first amino acid creates a bond with the nitrogen atom of the second amino acid. Since this reaction is a condensation reaction, hence it involves the release of water. As a result, a dipeptide molecule is created.
What is a Polypeptide?
When several amino acids are bonded together by a peptide bond, then the resulting molecule formed is known as a polypeptide. A protein may entail a single polypeptide chain, or it can include multiple chains that interact with each other.
Breakdown of Peptide Bonds
During the hydrolysis reactions, the peptide bonds are broken when water is added which results in the breakdown of polypeptides into amino acids.
In the next section of the article, we will discuss the four levels of protein structure in detail.
The Four Levels of a Protein Structure
Structure in proteins has four levels. Three of the four levels are associated with a single polypeptide chain, whereas the fourth level is associated with a protein that contains two or more polypeptide chains. Protein molecules of polypeptides can contain three to more than 34,000 amino acids that are bonded together in the form of chains. The example of a protein that has three amino acids is glutathione and the example of a protein that has more than 34,000 amino acids is a titan.
In the next section, we will discuss the primary structure of proteins.
Primary Structure of Proteins
The primary structure of proteins refers to the sequence of amino acids that are bonded by covalent peptide bonds. The primary structure of the protein is determined by the cell’s DNA. The DNA of the cell sends instructions to the cell to include specific amino acids in fixed quantities in a specific sequence. It influences the shape as well as the function of proteins. Each protein has a certain primary structure. Remember that a single alteration in the sequence of amino acids can affect the function of the protein.
Secondary Structure of Proteins
The interaction between the weak negatively charged oxygen and nitrogen atoms with the weal positively charged hydrogen atoms to form hydrogen bonds result in the secondary structure of proteins.
The hydrogen bonds can create the following two shapes within proteins:
-
- α-helix shape: It occurs when the hydrogen bonds are created between every fourth peptide bond, i.e. between the oxygen of the carboxyl group and the hydrogen of the amine group)
- β-pleated sheet: It is created due to the folding of proteins so that the two elements of the polypeptide chain are parallel to each other. This enables hydrogen bonds to create between parallel peptide bonds.
The majority of the fibrous proteins, for instance, keratin and collagen, have secondary structures. The secondary structure of only associated with the hydrogen bonds created between the amino group and the carboxyl group (the backbone of protein). High temperatures and changes in pH can break the hydrogen bonds.
Tertiary Structure of Proteins
Additional conformational alteration to the secondary structure results in the further formation of bonds between R groups, i.e., side chains.
The additional bonds include:
- Hydrogen (between R groups)
- Disulphide (these bonds only occur between cysteine amino acids)
- Weak interactions that are hydrophobic (between non-polar R groups)
- Ionic (these bonds occur between charged R groups)
The tertiary structure of proteins is common in globular proteins.
Quaternary Structure of Proteins
The quaternary structure of proteins occurs in proteins that contain more than one polypeptide chain that works together as a functional macromolecule. An example of such proteins includes haemoglobin.
Interactions and Shape of Proteins
The folding of the polypeptide chain occurs differently because of the interactions between the R groups. The three-dimensional configuration created is referred to as the tertiary structure of a protein.
Each amino acid entails a unique R group and hence several different interactions are possible. These interactions can create a wide range of protein configurations and functions. The following bonds are present within the tertiary structured proteins:
- Weak hydrogen
- Ionic
- Weak hydrophobic interactions
- Strong covalent disulphide
Disulphide bonds
Disulphide bonds refer to the strong covalent bonds that are created between two cysteine R groups. Although these bonds occur less frequently within the proteins, however, they are extremely strong. Disulphide bonds help to stabilize the proteins. These bonds are also referred to as disulphide bridges. Oxidation can break down these bonds and they are common in proteins that are secreted from the cells, for instance, insulin.
Ionic Bonds
Ionic bonds are created between positively charged and negatively charged R groups. They are not very common and are stronger than hydrogen bonds. The variation in pH can break these bonds.
Hydrogen bonds
Hydrogen bonds are created between R groups that are strongly polar. Hydrogen bonds are the weakest bonds but they are quite common because they are created between a wide range of R groups.
Hydrophobic interactions
Hydrophobic interactions are created between the non-polar R groups within the interior of proteins.
Summarise with AI:













Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead