Chapters
In this article, we will discuss how to carry out Benedict’s test for reducing sugars, the iodine test for starch, the emulsion test for lipids, and the biuret test for proteins. In addition to this, we will also describe the procedure to carry out a semi-quantitative Benedict’s test on a reducing sugar solution and identify the presence of non-reducing sugars, using acid hydrolysis and Benedict’s solution. So, let us get started.
In the laboratory, we can carry out certain tests to determine if the given sample has one of the key biological molecules such as carbohydrates, lipids, and proteins. The tests discussed here are qualitative in nature as they only tell us the type of biological molecule present in the sample rather than the quantitative value of the biological molecule in the sample.

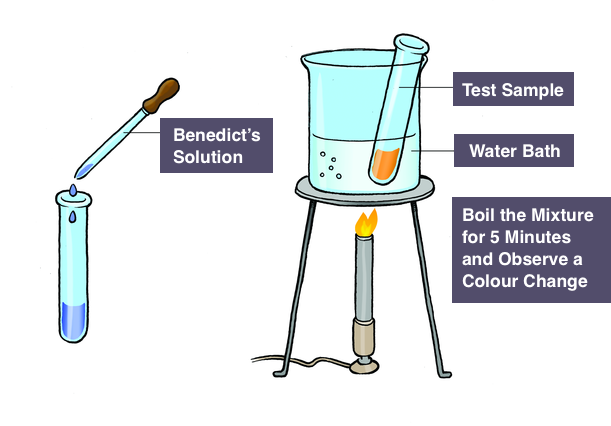
Benedict’s test for reducing sugars
Follow the steps below to carry out this test:
- Add Benedict’s reagent to the test tube containing the sample solution. (Benedict’s reagent is blue in colour because it contains copper (II) sulfate ions)
- Heat the test tube by using a water bath. Alternatively, you can also use the water beaker by bringing it to a boil for some minutes to heat the test tube.
Results
- In the presence of reducing sugar, you will observe the formation of a coloured precipitate because copper (II) sulfate is reduced to copper (I) oxide which is not soluble in water.
- Hence, we can conclude that the positive result is the change in colour from blue (no reducing sugar) to brown brick-red(indicating a higher concentration of reducing sugar).
We can say that the above test is semi-quantitative which means that the degree of a colour change indicates the concentration of reducing sugar present in the sample.
The Iodine Test for Starch
Follow the steps below to conduct this test:
- To determine whether the starch is present in a sample or not, add some drops of orange or brown iodine in potassium iodide solution to the sample (Since iodine is insoluble in water, hence it is in potassium iodide solution).
Results
- In the presence of starch, the iodide ions in the solution will react with the centre of starch molecules to produce a complex with a peculiar blue-black colour
- This test helps to show that starch in a sample is digested by enzymes
The Emulsion Test for Lipids
Lipids refer to nonpolar molecules that are insoluble in water but dissolve in organic solvents like ethanol.
- Add ethanol to the sample
- Shake the mixture and add it to a water test tube
Results
- A milky emulsion will form in the presence of lipids. It means that the solution will turn cloudy. The milky colour of the solution will be more prominent if more lipid is present
- The solution remains clear in the absence of lipids.
The Biuret Test for Proteins
- To conduct this test, treat the liquid solution of a sample with potassium or sodium hydroxide to make the solution alkaline
- Add some drops of copper (II) sulfate solution (blue) to the sample (Biuret reagent has copper (II) sulfate and an alkali)
Results
- The protein is present if you observe the change in colour from blue to purple/lilac. (The change in colour can be very subtle, so it is recommended to hold the test tube up against the white tile while observing the change n colour)
- No protein in the sample is present if no colour change is observed
For this test to be effective, there must be at least two peptide bonds present in any protein molecule. Hence, the test will be negative if the sample contains dipeptides or amino acids.
Semi-quantitative Benedict's Test for Reducing Sugars
- We can use Benedict’s solution to carry out a semi-quantitative test on a reducing sugar solution. The primary purpose of this test is to determine how much concentration of reducing sugar is present in the sample.
- It is essential to use an excess of Benedict’s solution so that there is sufficient copper (II) sulfate that can react with any sugar present.
- The intensity of any observed colour variation depends on the concentration of reducing sugar present in the sample.
- A positive test is shown along a colour spectrum from green (low concentration of reducing sugar) to brick-red (high concentration of reducing sugar).
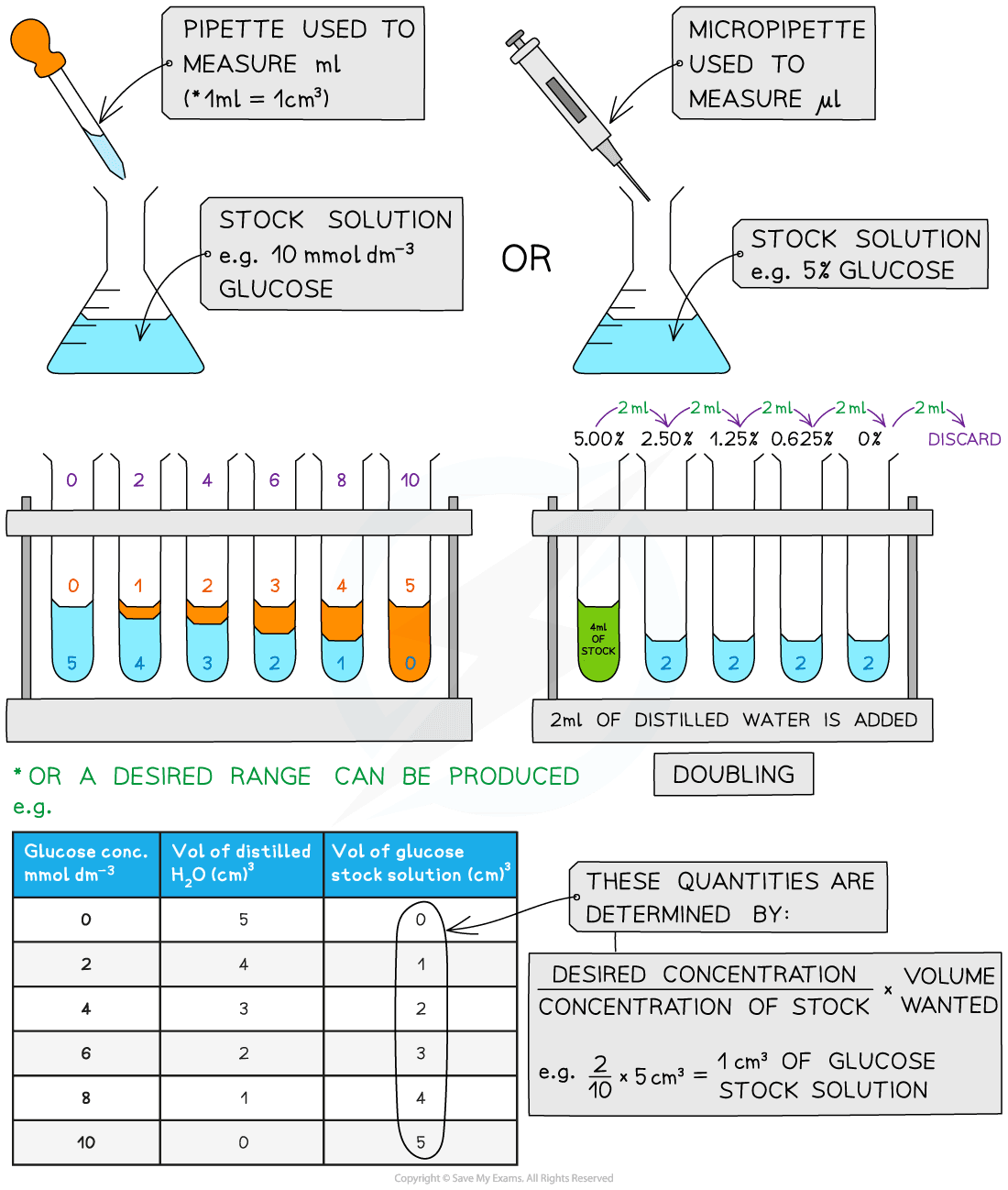
- We can carry out the semi-quantitative test by setting up standard solutions with familiar concentrations of reducing sugar, for instance, glucose.
- Set up this solution using a serial dilution of an existing stock solution.
- Treat each solution in the same manner, i.e. add the same volume of Benedict’s solution to each sample and heat it in the water bath that has been bought to the boil for a predetermined time (almost 5 minutes) to enable colour change.
- It is very important to use surplus Benedict’s solution.
- The colour change observed for each solution of a known concentration can be linked with the concentration of reducing sugar present in that solution.
- This procedure is carried out on a sample that contains unknown reducing sugar concentrations. After that, we can compare it to the stock solution colours to determine the concentration of reducing sugar present in the sample.
Modifications
- We can also standardize this test by measuring the time it takes for the first colour change to occur
- Colour change will be observed in less time if a higher concentration of reducing sugar is present in the sample
- To avoid human error in colour interpretations, a colourimeter can be employed to measure the absorbance or transmission of light through the sugar solutions of known concentrations
Serial Dilutions
- To create serial dilutions, take a series of dilutions of a stock solution. The concentration will reduce by the same quantity between each test tube
- They can be either “doubling dilutions (the concentration is halved between each test tube) or intended range (for instance, g. 0, 2, 4, 6, 8, 10 mmol dm-3)
- We complete the serial dilutions to create a standard to compare against which unknown concentrations can be compared.
- This comparison can be either visually measured through a standard curve or calibration or measured using a colourimeter.
- They can be used to enumerate bacteria or the population of yeast and to determine unknown concentrations of protein, starch, and glucose.
Testing for Non-reducing Sugars
We can classify sugars as reducing or non-reducing. This classification depends on the ability of sugars to donate electrons (a reducing sugar is oxidized if it can donate electrons).
Reducing sugars include:
- Glucose
- Galactose
- Maltose
- Fructose
Non-reducing sugars only include sucrose.
How to Test for Non-reducing Sugars?
- Add diluted hydrochloric acid to the sample and use a water bath to heat it after bringing it to boil
- Use sodium hydrogen carbonate to neutralize the solution.
- Utilize a suitable indicator (like red litmus paper) to determine when the solution has been neutralized. After that, add a little more sodium hydrogen carbonate because the conditions should be slightly alkaline for Benedict’s test.
- Carry out the normal Benedict’s test, i.e. add Benedict’s reagent to the sample and heat it in the boiling water bath. The colour variation indicates the presence of reducing sugar.
Important Points to Remember
- The acid is added to hydrolyze any glycosidic bonds present in any carbohydrate molecules.
- The remaining monosaccharides will have a ketone or aldehyde functional group that can donate electrons to copper (II) sulfate, enabling the formation of a precipitate.
Summarise with AI:













Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead