Chapters
In this article, we will investigate the progress of enzyme-catalyzed reactions by measuring rates of formation of products using catalase and rates of disappearance of the substrate using amylase. In addition to this, we will also outline the use of a colorimeter for measuring the progress of enzyme-catalyzed reactions that involve color changes. Before proceeding to discuss these points, first, let us discuss the enzymes and their structure.

What are Enzymes?
Enzymes refer to the protein substances that are required for all the biochemical reactions that occur in the living cells. They speed up (catalyze) the reactions that are necessary for life. Hence, they are referred to as biological catalysts.
Enzymes participate in the processes such as digestion and breakdown of nutrients, metabolism of nutrients to give energy, DNA, protein synthesis, and other syntheses of other biomolecules. They are also critical for the synthesis of membranes, cells, and tissues. Enzymes also participate in the processes of life like muscle contraction, neural functions, and motility, etc.
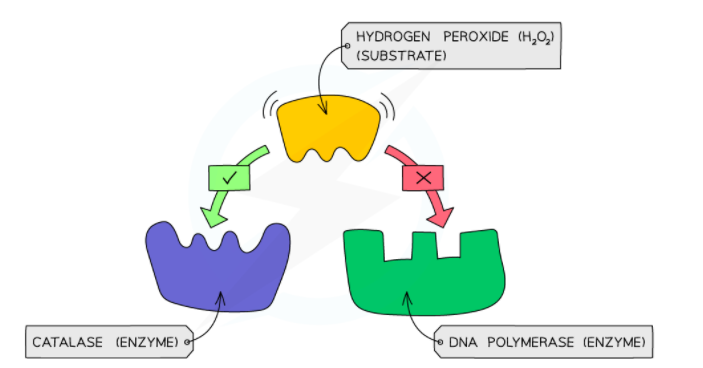
Enzymes speed up, i.e., catalyze the conversion of one or multiple substrates into a product during a chemical reaction. They also minimize the amount of energy needed for a chemical reaction to occur. Enzymes have high specificity for their substrate. To work properly, they require high optimum temperature and pH conditions. Two models: the lock and key hypothesis and the induced-fit model are used to explain the mechanism of enzymes action.
In the next section of the article, we will discuss the structure of enzymes in detail
Structure of Enzymes
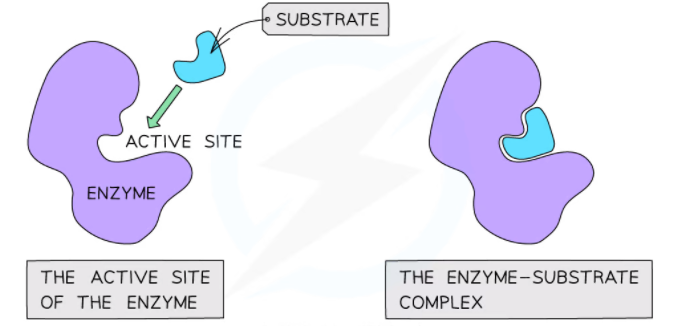
The majority of the enzymes are globular proteins that are composed of thousands of amino acids. The polypeptide side chains are folded onto themselves to create a compact globular structure. There is a cleft or space in each enzyme where the catalytic process occurs. This space or cleft is referred to as an active site or catalytic site.
Now, let us see what an active site is.
Active Site
An active site refers to a special cleft or space in an enzyme molecule that is created by the folding of the polypeptide chain. The substrate binds and the catalytic process occurs at this site. It is lined by amino acids that take part in the process of catalysis.
An enzyme-substrate complex is created when the substrate binds to the active site. The creation of the enzyme-substrate complex makes few changes in the active site that begin the catalytic process. An enzyme product complex is created when the process completes. The complex then sets apart, and the products of the reactions are released.
In the next section of the article, we will discuss how to measure enzyme activity.
How to Measure Enzyme Activity?
We can investigate the progress of enzyme-catalyzed reactions by:
- Measuring the rate of creation of a product using an enzyme known as catalase
- Measuring the rate at which the substrate disappears using an enzyme known as amylase.
In the next section, we will investigate the catalase activity.
Investigating Activity of the Catalase Enzyme
- In this experiment, the rate at which the product is formed is employed to measure the rate of enzyme-controlled reaction.
- The common but harmful by-product of metabolism is hydrogen peroxide. It implies that it should be broken down instantly to prevent any damage.
- The enzyme catalase is present in the cells of the majority of organisms.
- This enzyme breaks down the toxic hydrogen peroxide into oxygen and water.
- Catalase and hydrogen peroxide is combined, and the volume of oxygen produced is measured in a set time.
- Then we can compute the rate of reaction.
Now, let us see how to investigate the activity of an amylase enzyme.
Investigating activity of the amylase enzyme
- In this experiment, the rate at which the substance disappears is employed to compare rates of reaction under various conditions:
- The digestive enzyme that hydrolyses starch into glucose and maltose is known as amylase
- This enzyme works best at pH 7 and temperature 37oC (Remember that all the enzymes need specific conditions to work properly)
- After the starch and amylase are merged, the reaction mixture is tested for starch at definite time intervals.
- It can be achieved by taking samples from the mixture of reactions at each time interval and putting some iodine in potassium iodide solution in each sample(With this solution, the color of the starch turns blue-black)
- In this manner, the time taken to break down the starch can be calculated.
- We can repeat this investigation under a wide range of conditions such as changing temperature or pH. After that, we can compare the rates of reaction.
In the next section of the article, we will discuss how to use a colorimeter to measure the activity of an enzyme
Using a Colorimeter to Measure the Activity of an Enzyme
- With a colorimeter, we can measure the absorbance of light. The absorbance of light refers to the amount of light that is absorbed. We can also measure light transmission using this device which refers to the amount of light that passes through a substance.
- A colorimeter can be employed in any enzyme-catalyzed reaction in which the color change happens.
- The breakdown of color increases the transmission and decreases the absorption of light. This can be employed to measure the rate of reaction.
- For instance, the colorimeter can be employed to follow the development of a starch-amylase catalyzed reaction as amylase breaks down the starch into maltose.
- We can carry it out by colorimeter calibration and a calibration graph.
Colorimeter Calibration
In a colorimetric investigation, colorimeter calibration is a critical step. In this step, a weak iodine solution is employed to calibrate the colorimeter as the endpoint or 100% transmission.
It involves the preparation of a starch solution of familiar concentration from which many concentrations are made employing serial dilutions. The colorimeter is employed after calibration and switching on the red filter.
Calibration Graph
After that, a calibration graph is plotted of starch concentration of the x-axis and percentage transmission or absorption on the y-axis.
Summarise with AI:













Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead