Chapters
In this article, we will discuss the types of protein interactions in detail. But before proceeding to discuss the protein interactions, first, let us recall what are proteins.

What are Proteins?
Proteins form the basic structural component of all living things. Every living organism, whether it is big or as small as a microscopic organism, is made up of proteins. Proteins are essential for all the biochemical processes that are critical for the survival of an organism.
What are Proteins Made up of?
Proteins are one of the important macromolecules besides carbohydrates and lipids. They are polymers having a high molecular weight and are composed of structural units known as amino acids. The amino acids are the building blocks (monomers) of proteins. These organic compounds are composed of hydrogen, oxygen, nitrogen, and carbon atoms. A basic group known as an amino group, an acidic group known as a carboxyl group, a hydrogen atom, and a terminal R group collectively make up an amino acid. Different amino acids have different terminal R groups.
Proteins are composed of repeated units of amino acids which are attached to each other through peptide bonds. When two amino acids combine, a dipeptide bond is formed. A protein chain is made up of many amino acids known as a polypeptide.

The combination of similar amino acids is not essential in the formation of proteins. Hence, we can say that proteins can be formed in different ways, depending on the kind, order, and number of amino acids in the chain. The proteins such as alanine, glycine, and valine are made up of 20 amino acids.
In the next section of the article, we will discuss protein stability and denaturation of proteins.
Protein Stability and Denaturation
Proteins are highly sensitive macromolecules. We use the term “native state” to describe a protein that exists in its most stable natural form. External stress factors such as pH, temperature, water removal, and presence of metal ions, hydrophobic surfaces, and high shear can contribute towards disrupting the protein.
When a protein loses its secondary, tertiary, or quandary structure because of exposure to external stress, then we say that the protein has been denatured. Denaturing of protein causes the protein to unfold into a random or misfolded shape.
A denatured protein behaves differently than a protein in its native form. A denatured protein is usually unable to perform its biological function. Besides denaturation, the proteins can also create aggregates when exposed to some stress conditions. Aggregates are often the byproduct of a certain manufacturing process. They are undesirable because they can result in adverse immune responses when administered.
In the next section of the article, we will discuss proteins interaction and shape in detail.
Proteins Interactions and Shape
Because of the interactions between R groups, a polypeptide chain folds differently. The tertiary structure of the protein refers to the formation of a three-dimensional configuration. We already know that each of the twenty amino acids that participate in the formation of the proteins has a distinct R group. Hence, several different interactions are possible. These interactions can result in a wide range of protein configurations and functions.
The following bonds are present within tertiary structured proteins:
- Strong covalent disulphide
- Weak hydrophobic interactions
- Weak hydrogen
- Ionic
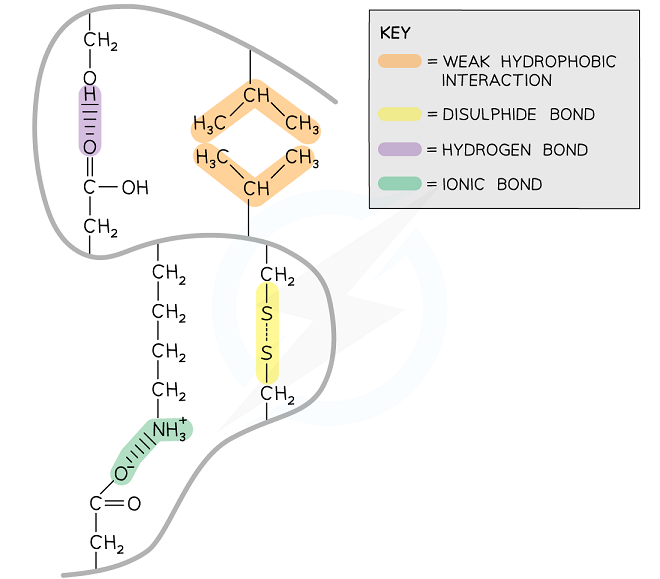
Strong Covalent Disulphide
Disulphide bonds refer to the strong covalent bonds that are present between two cysteine R groups. Although disulphide bonds occur less frequently between the proteins, however, they are the strongest ones. They are also referred to as disulphide bridges and contribute towards the stability of the proteins.
Oxidation can result in the breakage of disulphide bonds. They are common in proteins that are secreted from the cells, for instance, insulin.
Ionic bonds
Ionic bonds are created between the positively charged amine group and negatively charged carboxylic acid R groups. Although ionic bonds are stronger than hydrogen bonds, however, they are not very common. pH changes can break ionic bonds.
Hydrogen bonds
Hydrogen bonds are the weakest bonds that are created between strongly polar R groups. They occur more frequently because they are created between a wide range of R groups.
Hydrophobic Interactions
Hydrophobic interactions are created between the non-polar hydrophobic R groups within the interior of proteins.
In the next section of the article, we will discuss which protein bonds hold the primary, secondary, tertiary, and quaternary structures of proteins.
Bonds Present in Different Structures of Proteins
Protein structure has four different levels. Four of the three levels are attributed to one polypeptide chain and the fourth level is associated with a protein that contains two or more polypeptide chains. Proteins molecules or polypeptides can have three to more than 34,000 amino acids that are bonded together in chains.
Primary structure of proteins
The primary structure of the proteins is determined by the DNA of the cell which instructs the cell to include specific amino acids in certain quantities in a specific sequence. This influences the shape and hence the biological function of the protein. The amino acids in the primary structure of the proteins are bonded by covalent peptide bonds.
Secondary structure of proteins
The secondary structure of the protein occurs due to the interaction between weak negatively charged oxygen and nitrogen atoms with weak positively charged hydrogen atoms that create hydrogen bonds. The two shapes that can occur within proteins due to the presence of hydrogen bonds include α-helix and β-pleated sheets. The changes in pH and high temperatures can break the hydrogen bonds.
Tertiary Structure of Proteins
Additional conformational alteration of the secondary structure of proteins results in the further formation of bonds between R groups. These additional bonds include disulphide, hydrogen, ionic, and weak hydrophobic interactions. The tertiary structure of proteins is widely found in globular proteins.
Quaternary structure of proteins
The fourth level of the protein structure is known as the quaternary structure of proteins. This structure occurs in proteins in which more than one polypeptide chain works together as a functional macromolecule. An example of such protein includes hemoglobin. Every polypeptide chain in the quaternary structure is called a subunit of the protein.
Summarise with AI:












Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead