Chapters
In this article, we will discuss how hydrogen bonding takes place between water molecules and relate the properties of water to its roles in living organisms. We will specifically discuss solvent action, high specific heat capacity and latent heat of vaporization.
Water is critical for all living organisms. It forms the basis of life on this planet because it is the medium in which all the metabolic reactions occur in the cells. It is estimated that water constitutes almost 70% to 95% of a cell. Water is the primary habitat for living organisms, therefore, almost 71% of the earth’s surface is covered with water.

There are two atoms, i.e., hydrogen and oxygen in a water molecule. A water molecule is formed when one atom of hydrogen combines with two atoms of oxygen through covalent bonding (sharing of electrons). In the next section, how electron sharing happens in a water molecule.

How do atoms in water molecules share electrons?
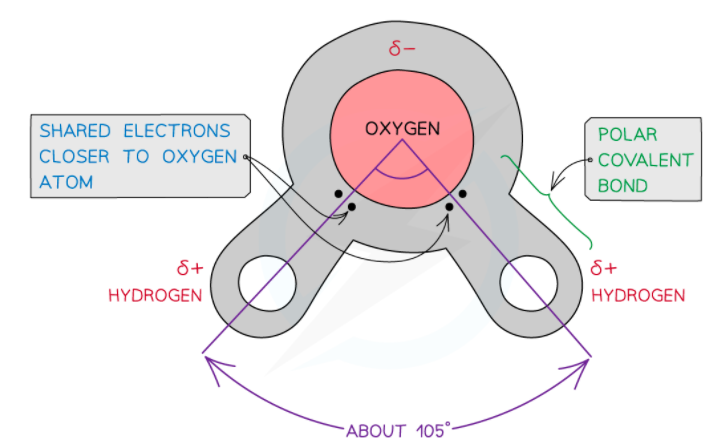
Water is electrically neutral and there is an uneven sharing of electrons between the hydrogen and oxygen atoms. This is because the oxygen atom in the water molecule attracts electrons more strongly as compared to hydrogen atoms. As a result, a weak negatively charged region on the oxygen atom and the weak positively charged region on the hydrogen atoms are formed which also results in the asymmetrical shape.
Why water is a polar molecule?
The charge separation because of the uneven sharing of electrons in the covalent bond is referred to as a dipole. When a molecule has one end that is negatively charged and another end that is positively charged, then it is a polar molecule. Since water molecule has two poles, i.e. a positive charge on the hydrogen end (pole) and the negative charge on the oxygen end (pole), hence it is referred to as a polar molecule.
Water as a General Solvent
Water is considered a general or universal solvent because of the polarity of its molecules. Because of the polarity of water molecules, several ions, for instance, sodium chloride, and substances that are covalently bonded (glucose) dissolve in it. This enables the chemical reactions to take place inside the cells. Dissolved solutes have greater chemical reactivity when they can move freely. This also helps the metabolites to move efficiently except for non-polar hydrophobic molecules.
All the substances that are critical for the survival of living organisms such as salts, vitamins, amino acids, glucose and gases move inside their bodies in the form of solutes that are dissolved in water. These substances participate in the metabolic reactions that occur inside the cells.
Hydrogen Bonds Between Water Molecules
Hydrogen bonds are created between water molecules. Due to the polar nature of water, hydrogen bonds are formed between positive and negatively charged regions of adjacent molecules. When there are limited hydrogen bonds, they are not only weak, but they also break and reform constantly. However, if there are a large number of hydrogen bonds, then they create a strong structure.
Hydrogen bonds participate in several properties of water molecules that are critical for living organisms. For instance, these bonds make water:
- A great solvent so that many substances can dissolve in water
- Have a relatively high specific heat capacity
- Less dense when it is solid
- Have high surface tension and cohesion
- Act as a reagent
Due to the above properties, water plays several important roles in living organisms. The properties of water discussed below are attributed to the presence of hydrogen bonds.
High Specific Heat Capacity of Water
The specific heat capacity of the substance refers to the amount of thermal energy needed to increase the temperature of 1kg of that substance by 1oC. The specific heat capacity of water is 4200 J/kg°C. Water has a high specific heat capacity because of the presence of several hydrogen bonds in it. It requires a great amount of thermal energy to break the strong hydrogen bonds in water. Similarly, a great amount of thermal energy is needed to build these bonds. Due to this reason, the water temperature does not fluctuate too much. Due to this property of water, it is of great importance for living organisms as it:
- Provides appropriate habitats
- Provides a constant temperature to living organisms because water can absorb much heat without big fluctuations in temperature. It is therefore critical to maintain temperatures that are necessary for the enzyme activity
- Water in blood plasms also transfers heat throughout the body and helps to maintain a constant body temperature.
- When blood moves through the parts of the body that are warmer (more active), heat energy is absorbed but the temperature remains nearly the same.
- Tissue fluid has water that plays a critical regulatory role in maintaining a consistent body temperature.
Latent Heat of Vaporization
To alter the state (from liquid to gas) water requires a huge amount of thermal energy so that it can be absorbed by the water and hydrogen bonds can break and evaporate. This is quite beneficial for living organisms because a small amount of water is needed to evaporate for an organism to lose a huge amount of heat. It provides a cooling effect to living organisms. For instance, when water evaporates from the surface of leaves due to transpiration, the temperature of leaves fall. Similarly, the bodies of human beings cool down when the water in sweat on the skin’s surface evaporates.
Cohesion and Adhesion
There is a strong cohesion between water molecules due to the presence of hydrogen bonds. Strong cohesion of water molecules help the living organisms in these ways:
- It enables water columns to move through the xylem of plants and blood vessels in animals
- It also helps surface tension at places where the water body meets air. These hydrogen bonds are present between a top layer of water molecules to form a kind of film on the water body.
Water also has the capacity to hydrogen bond with other molecules like cellulose. This is known as adhesion and it helps the water to move up in the xylem of plants due to transpiration.
Summarise with AI:













Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead