Chapters
Life depends on chemical reactions. From digesting your lunch to replicating DNA, thousands of processes are happening inside your cells right now. However, without help, these reactions would be far too slow to sustain life.
This is where enzymes come in. Enzymes are biological catalysts. Their job is to speed up the rate of chemical reactions without being used up or permanently changed themselves.
To understand how they achieve this, we need to look at their mode of action—the precise mechanism of how they bind to specific molecules and make reactions happen faster.

What Is Meant by the Mode of Action?
The "mode of action" describes the physical interaction between an enzyme and the substance it acts upon, known as the substrate.
Enzymes are globular proteins with a very specific 3D shape. Within this structure is a small, specialised area called the active site.
- The shape of the active site is determined by the enzyme's tertiary structure (how the protein chain folds up).
- Enzymes are specific: only a substrate with a complementary shape can fit into the active site.
When the substrate enters the active site, they join together temporarily. This crucial moment is what allows the reaction to occur.
Step-by-Step Mechanism
We can break the life cycle of an enzyme reaction into four distinct stages:
- Collision: Enzymes and substrates are usually moving randomly in a solution. A reaction can only happen if a substrate collides with the enzyme's active site at the right speed and angle.
- Formation of the Complex: The substrate binds to the active site. Temporary bonds (like hydrogen bonds) form to hold it in place. This structure is known as the enzyme-substrate complex.
- Catalysis: Once the complex is formed, the enzyme destabilizes the bonds within the substrate - lowering the activation energy for the reaction to occur. By putting strain on these bonds, the enzyme makes it easier for the substrate to break apart (catabolic) or join with another molecule (anabolic). This leads to the formation of an enzyme-product complex.
- Release: The reaction is finished. The product detaches from the active site and moves away. The enzyme remains unchanged and is immediately ready to accept a fresh substrate molecule.
Models of Enzyme Action
Scientists use models to visualise exactly how the substrate fits into the active site. Over time, our understanding has evolved from a rigid model to a more flexible one.
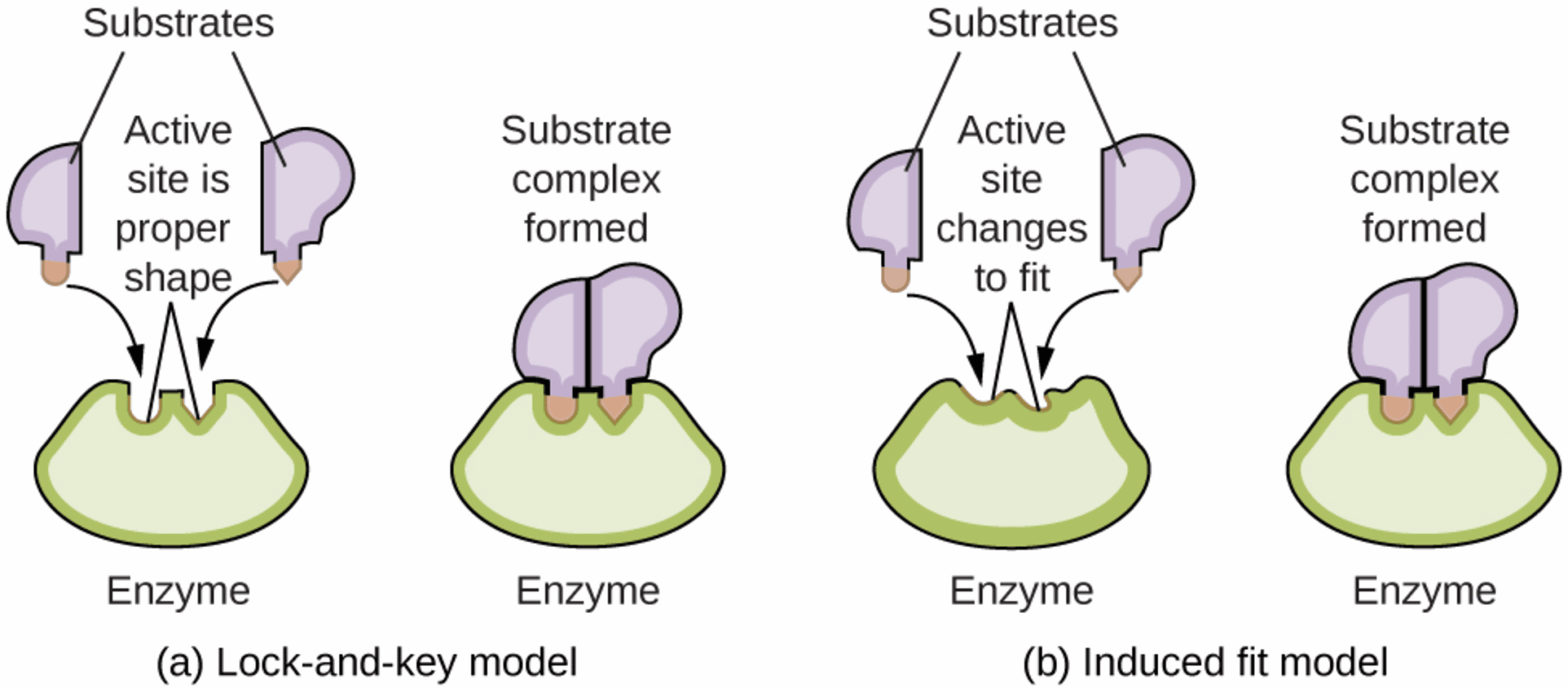
1. The Lock and Key Model - Proposed in the late 19th century, this model suggests a perfect, pre-existing fit:
- The enzyme is viewed as the lock (rigid and unchanging).
- The substrate is the key.
- The theory states that the active site is the exact complementary shape to the substrate before they even meet.
While this model explains specificity (why one enzyme only works on one substrate), it is now considered too simple.
2. The Induced-Fit Model - This is the currently accepted model. It suggests that enzymes are flexible structures, not rigid blocks:
- The active site is not a perfect fit initially.
- As the substrate enters, the enzyme changes its shape slightly to wrap around the substrate.
- This conformational change ensures a tighter fit, putting more strain on the substrate bonds and making the reaction more likely to happen.
Think of it like putting on a latex glove: the glove (enzyme) changes shape to fit your hand (substrate).
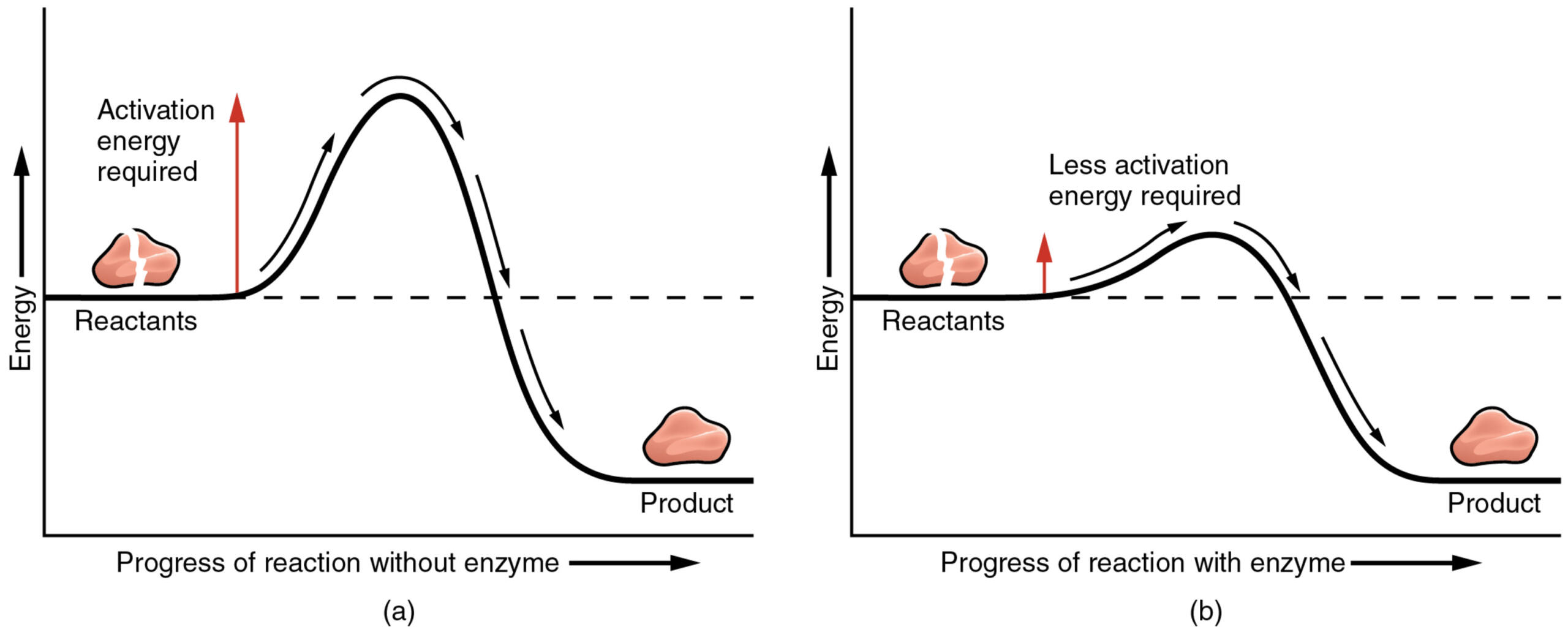
Lowering Activation Energy
For a chemical reaction to start, energy is required to break the existing bonds. This minimum energy threshold is called the activation energy.
If the activation energy is high, the reaction happens slowly (or not at all at body temperature). Enzymes work by providing an alternative pathway for the reaction that has a lower activation energy.
By holding the substrate in the optimal position and putting stress on its bonds, the enzyme reduces the energy needed to trigger the change.
Factors Influencing the Mode of Action
Since enzymes are proteins, their ability to function depends heavily on their environment.
- Temperature: Heat increases kinetic energy, leading to more collisions. However, if it gets too hot, the bonds holding the enzyme's structure together break. The active site loses its shape, and the enzyme becomes denatured.
- pH: Every enzyme has an optimum pH range. Deviating from this can disrupt the ionic bonds in the tertiary structure, affecting the shape of the active site.
- Inhibitors: These are molecules that slow down enzyme activity. Competitive inhibitors are shaped like the substrate and block the active site. Non-competitive inhibitors attach to a different part of the enzyme (allosteric site), causing the active site to warp so the substrate no longer fits.
Examples of Enzyme Mechanisms
1. Catalase (Intracellular): Hydrogen peroxide is a toxic substance produced naturally by cell metabolism. If left alone, it would damage cells. The enzyme catalase works inside cells to rapidly convert this toxin into safe products. The equation is:
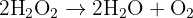
2. Amylase (Extracellular): Enzymes also work outside cells, particularly in digestion. Amylase is released by salivary glands in the mouth. It breaks down starch (a complex carbohydrate) into maltose (a simple sugar), starting the digestive process before food even reaches the stomach.
Summary
- Definition: Enzymes are biological catalysts that speed up reactions by lowering activation energy.
- Mechanism: Substrates bind to the active site to form an enzyme-substrate complex.
- Models: The Induced-Fit model (flexible) gives a more accurate description than the older Lock and Key model (rigid).
- Sensitivity: Enzyme activity is affected by temperature, pH, and inhibitors, which can lead to denaturation.
Summarise with AI:












Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead