Chapters
In this article, we will discuss respiration in anaerobic conditions in mammals (lactate fermentation) and yeast cells (ethanol fermentation). Moreover, we will also explain why the energy yield from respiration in aerobic conditions is much greater than the energy yield from respiration in anaerobic conditions. So, let us get started.
Initially, when life began on the planet earth, there was no oxygen for aerobic respiration. In other words, we can say that when life began, aerobic respiration was impossible due to a lack of oxygen. Hence, at that time, only anaerobic respiration occurred. Anaerobic respiration is a type of respiration that occurs in the absence of oxygen.
In anaerobic respiration, there is a net production of two ATP molecules per glucose molecule. Now, in eukaryotic cells, anaerobic respiration is employed as an emergency measure so that the functioning of important processes does not stop.
We can classify organisms into the following categories based on their dependence on oxygen:
- Obligate Anaerobes: These organisms are unable to survive in the presence of oxygen. This class is nearly exclusive to prokaryotes.
- Facultative Anaerobes: They can synthesize ATP through aerobic respiration; however, they can switch to anaerobic respiration when necessary. An example of such organisms includes yeast.
- Obligate Aerobes: They can only synthesize ATP if oxygen is present. We can classify a few individual cells in obligate anaerobes as facultative anaerobes; however, this is not permanent because it generates compounds that should be broken down when oxygen becomes available again.
In the next sections of the article, we will discuss respiration in anaerobic conditions in mammals (lactate fermentation) and yeast cells (ethanol fermentation).

Anaerobic Respiration
There are times when cells experience conditions in which little to no oxygen is available. The non-availability of oxygen for respiration has many consequences which are explained below:
- There is no final acceptor of the electron from the electron transport chain and the functioning of this chain stops
- ATP is no longer produced through oxidative phosphorylation
- The electron carrier does not oxidize reduced NAD and FAD
- There is no oxidized NAD and FAD available for dehydrogenation in the Krebs cycle which implies that the Krebs cycle stops
However, cells can still produce some ATP in conditions when oxygen availability is low via anaerobic respiration.
Anaerobic Pathways
Few cells can oxidize the reduced NAD generated during glycolysis so that it can be employed for further hydrogen transport. It implies that glycolysis does not stop and tiny amounts of ATP are still generated.
To achieve this, different cells employ different pathways. For example:
- Microorganisms and yeast employ ethanol fermentation
- Mammalian muscle cells and other microorganisms employ lactate fermentation
Fermentation
Fermentation refers to the process in which the organic compounds are broken down into smaller (simpler) inorganic compounds without the use of an oxygen or electron transport chain.
In this process, only a small quantity of ATP is generated by substrate-level phosphorylation. This is because, in fermentation, the glucose is not broken down as much as it is broken down in aerobic respiration.
When no oxygen is available to accept protons and electrons at the end of oxidative phosphorylation, then the synthesis of ATP by chemiosmosis stops. NADH and FADH2 are unable to oxidize because the flow of electrons stops. When the flow of electrons stops, there is nowhere for electrons to go. Hence, NAD and FAD are not regenerated. It implies that decarboxylation and pyruvate oxidation and the Krebs cycle stop because coenzymes are not available to eliminate the hydrogen ions.
Lactate fermentation generates lactate and takes place in animal cells. On the other hand, alcoholic fermentation produces ethanol and carbon dioxide and takes place in yeast and some plant cells.
Ethanol Fermentation
- In this pathway, the hydrogens from reduced NAD are transferred to ethanal to form ethanol.
- In the initial stage of this pathway, the pyruvate is decarboxylated to ethanal, thus producing carbon dioxide.
- Then the enzyme alcohol dehydrogenase reduces ethanal to ethanol
- Ethanal is the acceptor of hydrogen and it is unable to metabolize further. In short, it is a waste product.
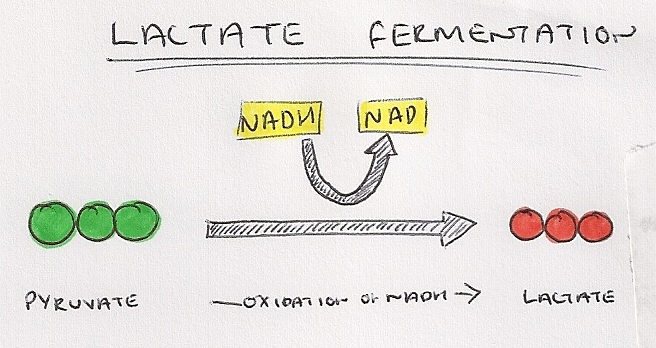
Lactate Fermentation in Mammals
- Pyruvate plays the role of the hydrogen acceptor as it accepts hydrogen ions from NADH, catalyzed by the enzyme lactate dehydrogenase
- Pyruvate is transformed into lactate or lactic acid and there is a regeneration of NAD.
- Lactate or lactic acid is transformed back into glucose in the liver, however, to complete this process, oxygen is needed. This is the reason behind oxygen debt after exercise.
- This can be employed to keep running the process of glycolysis to enable a small amount of ATP synthesis
- Lactate fermentation cannot occur indefinitely because of the following reasons:
- (sub arrow) The reduced amount of ATP generated would be insufficient to sustain important processes over time
- A fall in pH causes accumulation of lactic acid which can result in the denaturation of proteins. For instance, muscle fibres and respiratory enzymes stop functioning.
Lactate Metabolization
The following two things can happen after the production of lactate:
- It is able to oxidize back to pyruvate which is then channeled into the Krebs cycle for the production of ATP.
- It can be converted into glycogen which can be stored in the liver
The oxidation of lactate back to pyruvate requires surplus oxygen. This surplus oxygen is known as oxygen debt. Due to it, we breathe deeper and faster after strenuous exercise.
In the next section of the article, we will explain why the energy yield from respiration in aerobic conditions is much greater than the energy yield from respiration in anaerobic conditions.
Energy Yield in Aerobic and Anaerobic Respiration
- Energy yield from respiration in cells is much greater in aerobic conditions as compared to anaerobic conditions.
- In anaerobic respiration, glucose is only partially oxidized which implies that only some of its chemical potential energy is released and transferred to ATP. The only ATP-producing reaction that goes on is glycolysis.
- As no oxygen is available that can act as a final electron receptor, therefore no reactions can occur within the mitochondria. The stages that occur inside the mitochondria generate much more ATP than glycolysis.
Summarise with AI:












Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead