In this article, we will investigate the effects of immersing plant tissues in solutions of different water potentials. Moreover, we will also explain the movement of water between cells and solutions in terms of water potential and explain the different effects of the movement of water on plant cells and animal cells. So, let us get started.

Estimating Water Potential in Plant Tissue
Method
Follow the procedure below to investigate the effects of immersing plant tissues in solutions of varying water potential.
- Cut the required number of potato cylinders (cut one potato cylinder for each of the solutions you are testing. You can also cut more than one cylinder per solution if you intend to repeat the experiment)
- Cut all the potato cylinders into equal lengths. Then bottle-dry them to eliminate extra moisture. Measure and record the initial mass of the cylinder before putting them into solutions.
- Leave them in the solutions for a definite period, for instance, 30 minutes.
- After that, remove them from the solutions and dry them to remove extra liquid
- Measure and record the final length and mass of each potato cylinder
How to analyze results?
- Calculate the percentage change in mass for each potato cylinder
- If a potato cylinder has neither undergone an increase nor decrease in mass, then it implies that there was no overall net movement of water into or out of the potato cells
- This is due to the reason that the solution in which this particular potato cylinder was placed had the same water potential as the solution present in the cytoplasm of the potato cells. Hence, due to the absence of a concentration gradient, there was no net movement of water into or out of the potato cells.
- The sucrose concentration inside the potato cylinders can be calculated by plotting a graph that depicts how the percentage change in mass varies with the concentration of sucrose solution (The concentration of sucrose inside the potato cylinders is the point at which the line of best fir crosses the x-axis)
- A positive percentage change in mass implies that the potato has gained water through osmosis. It means that the water potential in the solution was higher than that of a potato.
- Due to the positive change in mass, the potato cells will become turgid because the water will exert a turgor pressure on the cell walls and make the potatoes hard.
- On the other hand, a negative percentage change implies that the water potential in the solution was lower than that of the potato
- The potato cylinder which was placed in the solution which contained the highest concentration of sucrose will be reduced in mass because there of the greatest concentration gradient in this tube between the potato cells and the sucrose solution
- It means that there will be more movement of water molecules out of the potato cells through osmosis which will make the cells flaccid and decrease the mass of the cylinders to make them feel floppy
- If we observe them under a microscope, the cells of the potato cylinder may be plasmolyzed which means that the cell membrane has moved away from the cell wall
In the next section, we will explain the different effects of the movement of water on plant and animal cells.
Osmosis in Plant Cells
Osmosis refers to the net movement of water molecules from an area of higher water potential, i.e., a dilute solution to an area of lower water potential, i.e. a concentrated solution through a cell membrane.
Let us now see how osmosis occurs in plant cells.
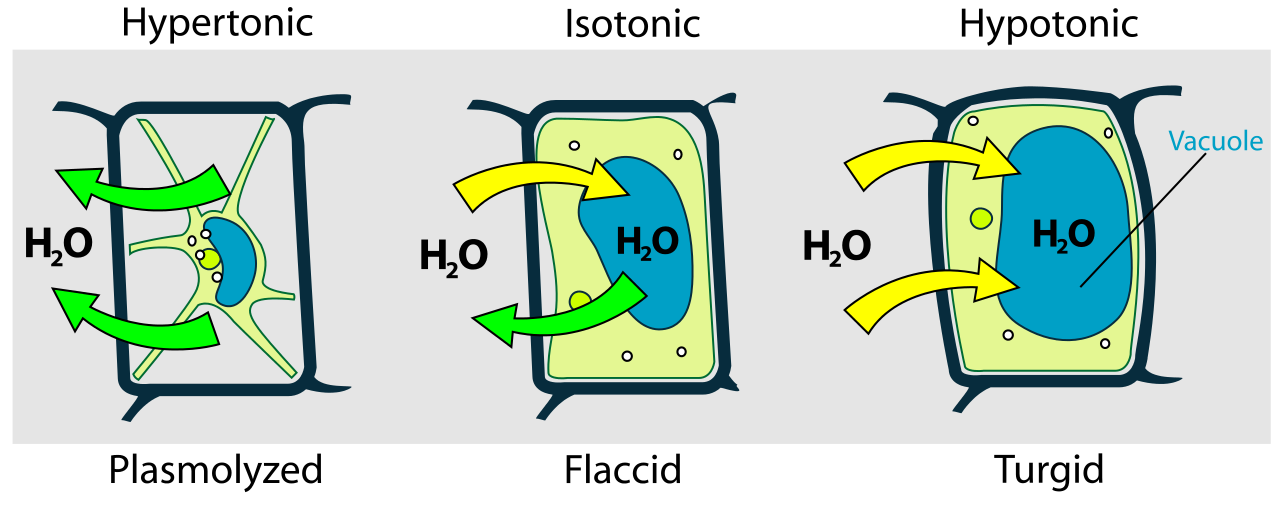
If we put a plant cell in a dilute solution or pure water, then the water will enter the cell through the cell membrane by osmosis because the pure water or dilute solution contains higher water potential than the plant cell. There is an increase in the volume of the plant cell as the water enters the vacuole of the cell. The pressure builds up inside the cell when the expanding protoplast pushes against the cell wall. The cell does not burst because it is surrounded by an inelastic cell wall.
Why is turgidity important for plant cells?
The plant cell is described as fully turgid when it becomes rigid and firm because it is inflated with water. The turgidity is critical for plants because the plant gains support and strength because their cells are firm. Due to this reason, plants stand upright with their leaves held out to obtain sunlight. In the absence of sufficient water, the cells in the plants cannot remain firm and rigid which can result in the wilting of the plant.
What happens when a plant cell is placed in a solution with a lower water potential?
When a plant cell is placed in a solution having a lower water potential than that of the plant cell, then the water will leave the cell through the cell membrane by osmosis. The volume of the cell reduces when the water leaves the vacuole of the plant cell. The protoplast shrinks slowly and does not exert any more pressure on the cell wall. The protoplast starts to pull away from the cell wall as it continues to shrink. This process is referred to as plasmolysis and we say that the plant has plasmolyzed.
Now, let us discuss how osmosis occurs in animal cells.
Osmosis in Animal Cells
Like plants, animal cells also gain and lose water due to osmosis. However, unlike plant cells, animals cells do not have supporting cell walls. Due to this reason, the gain and loss of water in an animal cell are more severe compared in the plant cells.
What happens when an animal cell is placed in a concentrated solution?
If we put an animal cell in a solution that contains lower water potential than the cell, then the water will leave the cell through the cell membrane by osmosis. As a result, the cell will shrink and shrivel up. This happens when a cell is in a hypertonic environment, i.e. the solute concentration in the solution outside the cell is higher than inside the cell.
What happens when an animal cell is placed in a dilute solution?
If an animal cell is placed in a dilute solution or pure water, then the water will enter the cell through the cell membrane by osmosis. This is because the pure water or dilute solution contains a higher water potential. The cell will continuously gain water through osmosis until the cell membrane is pushed far enough and the cell bursts. The cell bursts because the supporting cell wall is absent in the animal cells. It happens when the cell is in a hypotonic environment which means that the solution outside the cell has a lower concentration of solute than the solution inside the cell. Therefore, it is critical to maintaining a consistent water potential inside the animal bodies.
If the animal cell is placed in the isotonic environment which means that the solution outside the cell has a lower concentration of solute than inside the cell, then the water will move into and out of the cells at the same rate and the cell will not undergo any change because of zero net movements of water molecules.
Summarise with AI:













Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead