Chapters
Nucleotides refer to the biological molecules that act as a foundation for nucleic acids such as DNA and RNA. They are critical for all the functions carried out by a living cell. Besides this, they are also important for the transfer of information to new cells or to the next generation of living organisms.
Nucleotides combine to form dinucleotides, tri-nucleotides, and so on. It means that the combination of nucleotides gives rise to the polymers called polynucleotides. These polynucleotides combine to create complicated nucleic acids such as DNA and RNA.
In the next section of the article, we will discuss the nucleotide structure in detail.

Structure of Nucleotides
- Nucleic acids like DNA and RNA are giant molecules, i.e. macromolecules
- These nucleic acids are polymers like carbohydrates (polysaccharides) and proteins (polypeptides). It implies that they are composed of several smaller, similar molecules that are combined for a long chain. These smaller units are referred to as monomers or subunits
- Hence, DNA and RNA are also called polynucleotides
In the next section of the article, we will explain the composition of nucleotides in detail.
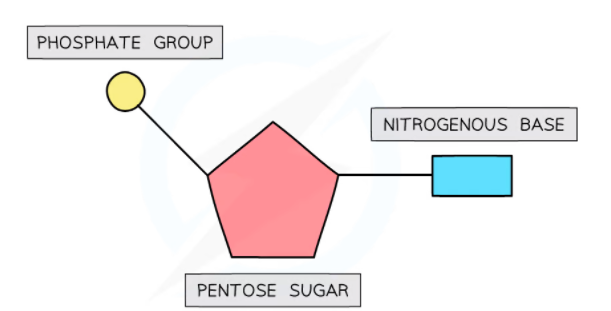
Composition of Nucleotides
Nucleotides are composed of the following three components:
- A pentose sugar (pent means five): This name is given as it contains five carbon atoms
- A nitrogen-containing base which is also referred to as a nitrogenous base
- A phosphate group
Pentose sugar
As the name implies, a pentose sugar refers to a monosaccharide that contains five carbon atoms. All other parts of the nucleotide are connected with the carbon atoms of the pentose sugar. The two types of pentose sugar found in the nucleotide structure are:
- Ribose
- 2-Deoxy ribose
The primary difference between these two sugars lies in the presence or absence of oxygen atoms at the second carbon atom in the structure. There is a hydroxyl group at the second carbon atom in the ribose sugar, whereas a single hydrogen atom at the second carbon atom in the 2-deoxy ribose. Both ribose and 2-deoxy ribose occur in the form of a ring within a nucleotide.
Nitrogenous Bases
Nitrogenous bases refer to the nitrogen-containing molecules that act as a base. They are one of the important components of nucleotides. Based on the structure of nitrogenous bases, they are divided into the following two broad categories:
- Purines
- Pyrimidines
Purines contain two rings in their structure that are composed of carbon and nitrogen atoms. The larger ring is hexagonal (having six sides) in structure, whereas the smaller ring is pentagonal (having five sides) in structure. Adenine and Guanine are the two important purines present in the nucleotides. They contain a double ring structure.
Pyrimidines contain a single ring that is composed of both carbon and nitrogen atoms. Their ring is hexagonal in structure. The following three purines are essential in biological molecules:
- Cytosine
- Thymine
- Uracil
The bases cytosine, thymine, and uracil have a single ring structure.
A single nitrogenous base is joined with the first carbon of a pentose sugar to create a nucleoside. It becomes a nucleotide when a phosphate group is added to it.
Phosphate Groups
Phosphate groups are another important component of a nucleotide. They are phosphate ions composed of a phosphorous atom that is bound to four oxygen atoms 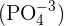 . They contain a negative charge of -3. The first phosphate group is joined with the fifth carbon of the pentose sugar through an ester bond. The next phosphate group is joined with the first phosphate and so on. The nucleotide that contains a single phosphate group is known as monophosphate nucleotide and so on.
. They contain a negative charge of -3. The first phosphate group is joined with the fifth carbon of the pentose sugar through an ester bond. The next phosphate group is joined with the first phosphate and so on. The nucleotide that contains a single phosphate group is known as monophosphate nucleotide and so on.
In the next section of the article, we will discuss the functions of nucleotides.
Functions of Nucleotides
Nucleotides perform many critical functions not only in their free form but also as a component of nucleic acids. Being a component of nucleic acids, the nucleotides play an important role in:
- Transmission of genetic information from one generation to the next
- Transmission of information from the nucleus to the cytoplasm
- Protein synthesis
- Cell cycle control
In the free form, the nucleotides perform the functions listed below:
- They provide energy for different metabolic processes in the form of ATP, GTP, etc
- They take part in cell signalling. For instance, the role GTP plays in G-protein coupled receptors
- They play the role of cofactors for enzymes like NAD, NADPH, etc.
In the next section, we will discuss what is ATOP.
What is ATP?
ATP which stands for Adenosine triphosphate refers to the energy-carrying molecule that provides energy to drive several important processes inside living cells. ATP is structurally quite similar to the nucleotides that participate in the composition of DNA and RNA, hence it is considered another kind of nucleic acid. ATP is a phosphorylated nucleotide. Adenosine (single nucleotide) is combined with one, two, or three phosphate groups:
- When adenosine is combined with one phosphate group, then it is called adenosine monophosphate (AMP)
- When adenosine is combined with two phosphate groups, then it is called adenosine diphosphate (ADP)
- When adenosine is combined with three phosphate groups, then it is called adenosine triphosphate (ATP)
In the next section, you will find a summary of the entire article.
Summary of the Article
- Nucleotides refer to the biological molecules that act as a foundation of nucleic acids. They are present in DNA and RNA.
- Three components such as a pentose sugar, a nitrogenous base, and a phosphate group make up a nucleotide.
- The pentose sugar is the primary component and nitrogenous base, and phosphate groups are attached to it.
- Nitrogenous bases are of two types: purines (double-ring structure) and pyrimidines (single ring structures)
- Adenine and guanine are included in the purines
- Cytosine, thymine, and uracil are included in the pyrimidines
- A nucleoside is formed by combining a nitrogenous base and a pentose sugar
- When a phosphate group is added to a nucleoside, a nucleotide is formed
- A nucleotide can contain one, two, or three phosphate groups
Summarise with AI:













Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead