Chapters
In this article, we will discuss what is reducing and non-reducing sugars, and which tests are used to determine whether the given sample contains a reducing or non-reducing sugar. So, let us get started.
Sugars can be classified into the following two groups based on their ability to donate electrons:
- Reducing sugars
- Non-reducing sugars

Reducing sugars
Reducing sugars have the ability to donate electrons. The carbonyl group in these sugars gets oxidized and the sugars become the reducing agent. Hence, we can use Benedict’s test to identify the reducing sugar in the given sample because if these sugars are present in the sample, they reduce the soluble copper sulfate to insoluble copper oxide which is of brick-red colour.
Examples of reducing sugars include glucose, fructose, and maltose.
Non-reducing sugars
Since non-reducing sugars do not possess the ability to donate electrons, hence they cannot be oxidized. To identify a non-reducing sugar in a sample, it must be hydrolyzed first to break disaccharides into two monosaccharides before carrying out Benedict’s test.
An example of non-reducing sugar includes sucrose.
In the next section of the article, we will discuss the biochemical tests that can be carried out in the laboratories to identify the reducing sugars in a given sample
Biochemical Tests to detect Reducing Sugars
- Several tests can be carried out in the laboratories to detect the presence of a certain kind of sugar in the given sample.
- The tests that we will discuss here are qualitative. It means that they do not yield a quantitative value depicting how much of a specific molecule is present in the sample being tested.
In the next section, we will discuss how to carry out Benedict’s test for reducing sugars (sugars that can donate electrons and are oxidized).
Test for Reducing Sugar – The Benedict’s Test
- Benedict’s reagent is used in Benedict’s test which is a blue solution that has copper (II) sulfate ions (
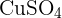 ). If reducing sugar is present, then copper (I) oxide is created.
). If reducing sugar is present, then copper (I) oxide is created. - Since copper (I) oxide is insoluble in water, therefore it forms a precipitate
Follow the procedure below to carry out Benedict’s test for reducing sugar.
Procedure
- Put the sample solution in a test tube and add Benedict’s reagent. [Benedict’s reagent is a blue-coloured solution because it contains copper (II) sulfate ions]
- Bring the water bath or water beaker to boil for a few minutes and heat the test tube in it
- In the presence of reducing sugar, you will observe the formation of coloured precipitate because copper (II) sulfate is reduced to copper (I) oxide which is not soluble in water
- It is critical to use an excess of Benedict’s solution to ensure the presence of sufficient copper (II) sulfate that can react with any sugar that is present in the sample
- A positive test result will indicate a change in a colour somewhere along a colour scale from blue (indicating no reducing sugar), through green, yellow and orange (indicating the presence of low to medium concentration of reducing sugar) to brown or brick-red (indicating the presence of a higher concentration of reducing sugar)
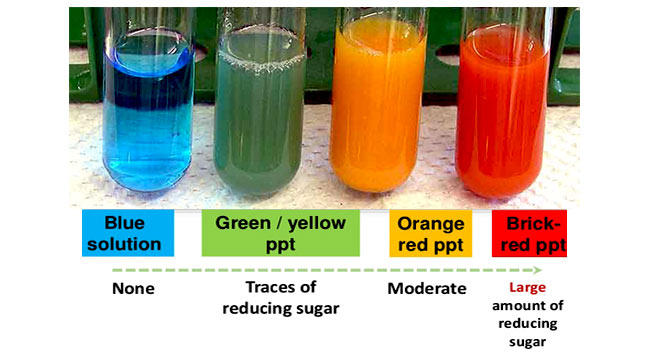
Remember that this test is semi-quantitative because the degree of a colour change indicates the amount (how much concentration of) reducing sugar is present in the given sample.
In the next section, we will discuss how to carry out the test for the presence of non-reducing sugar in a given sample.
Testing for Non-reducing sugars
Follow the procedure below to test the presence of non-reducing sugar in a given sample.
Procedure
- Add dilute hydrochloric acid to the sample being tested and heat it in a water bath that has been brought to boil for some minutes
- Use sodium hydrogen carbonate to neutralize the solution
- Employ an appropriate indicator (for instance, a red litmus paper) to determine when the solution has been neutralized. Then, add a little more sodium hydrogen carbonate because the conditions should be a bit alkaline for Benedict’s test to work.
- After that, carry out Benedict’s test as normal (Add Benedict’s reagent to the sample under test and heat it in a water bath that has been brought to boil for some minutes. The colour change will indicate the presence of reducing sugar).
Explanation
- The acid is added because it will hydrolyze any glycosidic bonds that are present in any carbohydrate molecules
- The monosaccharides left behind will have an aldehyde or ketone functional group that has the ability to donate electrons to copper (II) sulfate (reducing the copper), enabling the formation of a precipitate.
In the next section of the article, we will discuss the iodine test for starch. But before discussing how to carry out this test, first, let us recall what is starch.
What is Starch?
- Starch refers to the storage polysaccharides of the plants.
- Starch is stored as granules in plastids (for instance, chloroplasts)
- It takes more time to digest starch as compared to glucose because of the presence of several monomers in a starch molecule
- Starch is made from two distinct polysaccharides: Amylose and amylopectin
Amylose (It contains 10 to 30% of starch)
- Amylose contains an unbranched helix-shaped chain that has 1,4 glycosidic bonds between alpha glucose molecules
- The helix shape of this polysaccharide allows it to be more compact and hence more resistant to digestion, i.e., it makes it hard to digest
Amylopectin (It contains 70 to 90% of starch)
- It has 1,4 glycosidic bonds between α-glucose molecules as well as 1,6 glycosidic bonds created between glucose molecules to create a branched molecule.
- The branches result in several terminal glucose molecules that can be hydrolyzed easily so that they can be used during cellular respiration or added to for storage purposes.
In the next section, we will discuss the iodine test for starch.
The Iodine Test for Starch
- To determine whether starch is present in a given sample, add some drops of orange or brown iodine in potassium iodide solution to the sample
- We add iodine in potassium iodide solution because iodine is not soluble in water
- In the starch is present in a sample, the iodide ions in the solution react with the centre of starch molecules to produce a composite that has a unique blue-black colour
This test helps in experiments to depict that starch in a sample is digested by enzymes.
Summarise with AI:













Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead