Chapters
Oxidative phosphorylation through the chemiosmotic theory occurs on the inner membrane of the mitochondria and needs NADH and FADH2 from the Krebs cycle which is also known as a citric acid cycle. As a result of this process, water and several molecules of ATP are generated. In this article, we will discuss oxidative phosphorylation in detail. So, let us get started.

Oxidative Phosphorylation Explained
The fourth and final step of cellular respiration is referred to as oxidative phosphorylation. It is an aerobic process as it requires oxygen. Oxidative phosphorylation is the primary producer of ATP in the process. Two elements that constitute oxidative phosphorylation include the electron transport chain and chemiosmosis.
Electrons in an electron transport chain move from one carrier to another to create an electrochemical gradient. This electrochemical gradient can be employed to power oxidative phosphorylation. Chemiosmosis refers to the creation of ATP using the electrochemical gradient. About 28 ATP molecules are produced by this process which is a lot more than the process of glycolysis.
In the next section, we will discuss the electron transport chain in detail.
The Electron Transport Chain
The electron transport chain constitutes part of the last stages of aerobic respiration. A series of redox reactions are involved in the electron transport chain. In these reactions, the electrons are moved between membrane-spanning proteins. Oxygen is the last link in the chain which is the final acceptor of electrons. Electrons reduce oxygen to create water. The electron transport chain is unable to run in the absence of oxygen and no more ATP is produced by the chemiosmosis. Cells are unable to carry out reactions if ATP is insufficient.
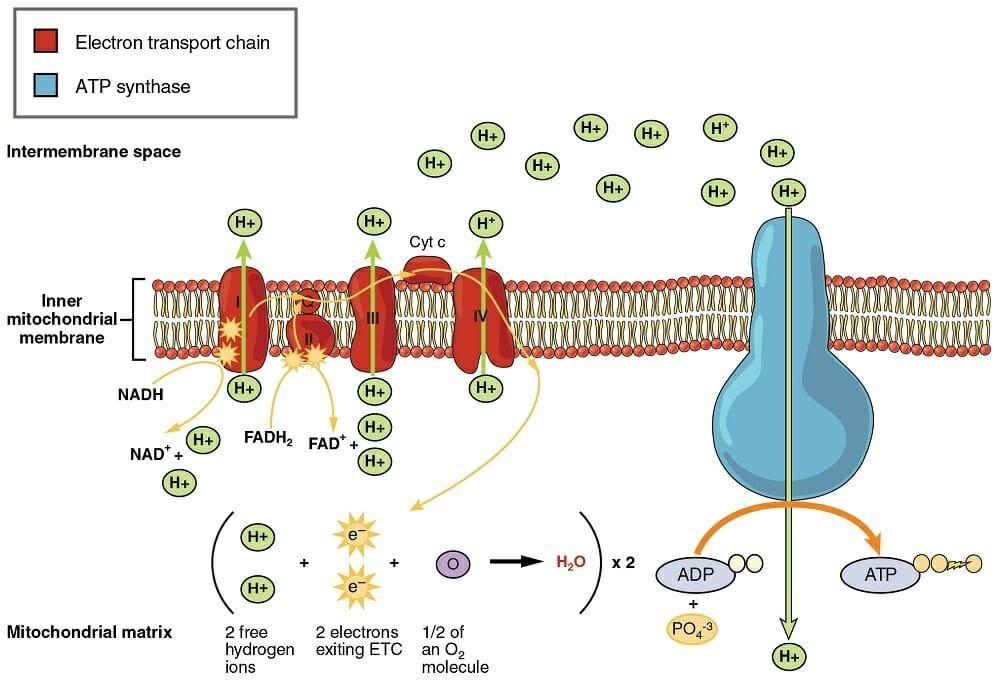
The elements of the electron transport chain are categorized into four large complexes which are labeled from I to IV. Electrons enter the electron transport chain from FADH2 and NADH molecules. These two molecules are produced during the preliminary steps of cellular respiration. When the electrons move across the electrochemical gradient and many protein complexes employ this released energy to pump protons from the mitochondrial matrix to the intermembrane space, a proton gradient is established, and energy is released.
In the next section, we will discuss what happens in an electron transport chain.
What Happens in the Electron Transport Chain?
First, the electron carriers NADH and FADH deliver the electrons that enter the electron transport chain. The reduced electron carriers from the past steps of cellular respiration move their electrons to the molecules near the start of the transport chain. When these electron carriers NADH and FADH lose their electrons, they are oxidized into NAD+ and FADH. They can be recycled further to other steps of respiration.
The process begins when NADH deposits its electrons at Complex I to form NAD+ and releases a proton into the matrix. Since the electrons of FADH2 have a lower energy level as compared to NADH, therefore it cannot donate electrons like NADH. It means FADH2 is unable to transfer its electrons to Complex I.
FADH2 deposits its electrons at Complex II and is then reduced to FAD and releases two hydrogen atoms. The electrons from both the complexes, i.e., Complex I and Complex II are then moved to another carrier known as ubiquinone (Q). The reduced form of Q is QH2 which is a mobile electron carrier that moves freely through the membrane. The electrons from Q are passed to Complex III. As electrons move through Complex III, a greater number of hydrogen ions are pumped across the membrane. The electrons are transferred to cytochrome C which is also mobile and passes freely through the membrane.
Cytochrome C moves the electrons to Complex IV which then transfers the electrons to a terminal electron acceptor oxygen. Oxygen is divided into two oxygen atoms and accepts hydrogen ions from the matrix to create water. A single water molecule is created from two electrons, one oxygen (half  molecule), and two hydrogen ions. Electrons release energy as they pass from higher to lower energy levels to move protons out of the matrix and into the intermembrane space. This energy is employed by complexes I, III, and IV.
molecule), and two hydrogen ions. Electrons release energy as they pass from higher to lower energy levels to move protons out of the matrix and into the intermembrane space. This energy is employed by complexes I, III, and IV.
In the next section, we will discuss the chemiosmosis in oxidative phosphorylation in detail.
Chemiosmosis and Oxidative Phosphorylation
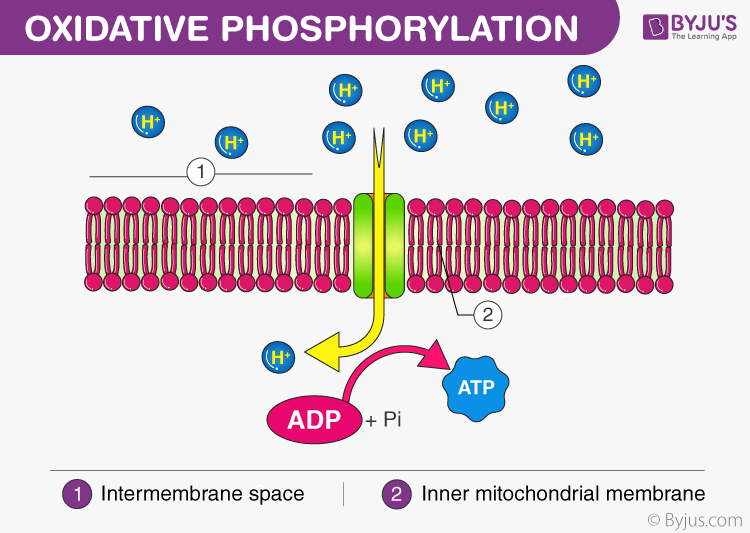
A proton gradient is created when the electrons move through the electron transport chain. A protons gradient is created due to the accumulation of positively charged hydrogen ions at one side of the membrane. This proton is then employed to power ATP synthesis.
A membrane-embedded protein known as ATP synthase helps the hydrogen ions in the mitochondrial matrix to move through the phospholipid bilayer of the inner membrane. The working of ATP synthase resembles that of a water wheel, i.e., it is turned through the flow of hydrogen ions passing through it, down their electrochemical gradient.
The turning of ATP synthase catalyzes the addition of phosphate to ADP to create ATP. This process in which energy from a proton gradient is employed to create ATP is referred to as chemiosmosis.
Production of ATP
As a result of cellular respiration in the eukaryotic cell, 30 to 32 ATP molecules per glucose molecule are generated. The majority of the ATP molecules are produced by the electron transport chain. On the other hand, the process of glycolysis generates only 2 ATP molecules. The electron transport chain is quite efficient at generating energy in the cell; however, it requires abundant oxygen to do so.
In the next section of the article, you will find the summary of oxidative phosphorylation.
Summary of Oxidative Phosphorylation
The main points discussed in this article are summarized as follows:
- Oxidative phosphorylation is constituted of the electron transport chain and chemiosmosis.
- In the process of aerobic respiration, oxidative phosphorylation is one of the most efficient producers of ATP molecules.
- Electrons carried from the last stages of respiration enter the electron transport chain and are moved sequentially through proteins that are bounded by a membrane.
- Oxygen is the last member of the chain which creates water when it accepts the electron.
- A proton gradient is established as a result of the electron transport chain which further runs ATP synthase activity.
Summarise with AI:













Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead