Chapters
In this article, we will discuss factors that affect enzyme action. Besides this, we will also discuss that the maximum rate of reaction (Vmax) is used to derive the Michaelis–Menten constant (Km), which is employed to compare the affinity of different enzymes for their substrates. So, let us get started.
Enzymes refer to the biological catalysts that speed up (catalyze) the chemical reactions taking place inside our bodies.

Factors Affecting Enzyme Activity
Various factors such as temperature, ph, enzyme, substrate, and inhibitor concentration affect enzyme activity.
1. Temperature
Enzymes require a certain optimum temperature to function properly. This is the temperature at which they can speed up the chemical reaction at the maximum rate.
Effects of lower temperature
Lower temperatures either stop the reactions completely or slow them down. This is because the movement of molecules is relatively slower which can result in a lower number of successful collisions between the substrate molecules and the active site of enzymes. As a result, there will be less formation of the enzyme-substrate complexes. When substrate and enzyme collide with each other with less energy, then there is a lower probability for bonds to be created or broken which can stop the reaction altogether.
Effects of higher temperature
Higher temperatures speed up the reactions as they result in a higher number of successful collisions between the active site of enzyme and substrate molecules. This will result in a more frequent creation of enzyme-substrate complex. As substrate and enzyme collide with greater energy, the bonds are more likely to be created or broken which enables the reactions to occur. If the temperature increases continuously, then the rate at which the enzyme speeds up the reaction drops significantly and denaturing of the enzyme occurs.
What is denaturing?
Denaturing starts when the bonds that hold the enzymes in their accurate shape begin to break. This alters the tertiary structure of the protein which further damages the active site permanently and prevents the binding of the substrate. If the substrate cannot bind, then denaturation occurs.
Only a small number of enzymes in human bodies can function at temperatures above 50oC. This is because humans maintain a body temperature of about 37oC and the denaturation of enzymes occurs even at temperatures exceeding 40oC. Hydrogen bonds between the amino acids start to break at higher temperatures, altering the enzyme configuration.
2. pH
Enzymes require a certain pH at which they can function properly. Extremes of pH can result in the denaturation of enzymes. The tertiary structure of the protein (enzyme) is held by ionic and hydrogen bonds. If the pH falls below or above the optimum pH required by the enzyme, then the acidic and alkaline solutions can cause the breakage of the bonds. This further results in the change in the shape of the active site which means that the enzyme-substrate complexes cannot be created easily. Eventually, enzyme-substrate complexes cannot be created at all. The final outcome is the denaturation of the enzyme.
The function of an enzyme is an indicator of its optimal environment. For instance, the optimum pH of the enzyme pepsin present in the stomach is 2 because the stomach has an acidic environment due to the presence of hydrochloric acid in the stomach’s gastric juice.
Investigating the effect of pH on the enzyme-catalyzed reaction
We can employ buffer solutions to investigate the impact of pH on the rate of an enzyme-catalyzed reaction. This solution is used to measure the rate of reaction at various pH values. Each of the buffer solutions has a certain pH which they maintain even if the reaction occurring would result in a change in the pH of the reaction mixture.
In this experiment, a specific volume of the buffer solution is added to the reaction mixture. For each pH value that is being investigated, the same volume of each buffer solution used is added.
3. Enzyme Concentration
The rate of the reaction is affected by the enzyme concentration. If the enzyme concentration in a reaction mixture is higher, then it will result in a higher number of available active sites. There will be also more probability of the creation of an enzyme-substrate complex. Until enough substrate is available, the initial rate of reaction increases linearly with the enzyme concentration.
If the substrate amount is limited, then after a certain point, any further increase in the concentration of enzyme will not enhance the rate of reaction because the substrate amount becomes the limiting factor.
4. Substrate Concentration
The rate of reaction is higher if there is more concentration of substrate. The probability of the formation of enzyme-substrate complexes increases with the increase in the number of substrate molecules. If the enzyme concentration remains constant but the substrate amount increases beyond a certain limit, then all the active sites become saturated and an additional increase in the concentration of substrate will not increase the rate of reaction.
When all the active sites of the enzymes are full, then any substrate molecules added have no active sites to bind with to create enzyme-substrate complexes.
5. Inhibitor Concentration
Inhibitors are of two types:
- Competitive: The shape of these inhibitors is the same as of the substrate molecules, hence they compete with the substrate for the active site. For competitive inhibitors, we can counter the increase in the concentration of inhibitors by increasing the concentration of the substrate. This will increase the rate of reaction.
- Non-competitive: These inhibitors bind to the enzyme at an alternative site which changes the shape of the active site and stops the substrate from binding to it. For non-competitive inhibitors, we cannot increase the reaction rate by increasing the concentration of the substrate. This is because of the change in the shape of the active site which prevents the formation of enzyme-substrate complexes.
Both types of inhibitors: competitive and non-competitive slow down or prevent the action of the enzyme. When the concentration of an inhibitor increases, the rate of reaction decreases. If the concentration of the inhibitor continues to increase, the reaction will stop altogether.
Vmax & the Michaelis-Menten Constant
The concentration of the substrate influences the catalysis rate in an enzyme-substrate reaction. When the concentration of a substrate is constant, then the initial reaction rate is the fastest. The rate of reaction declines as active sites become engaged.
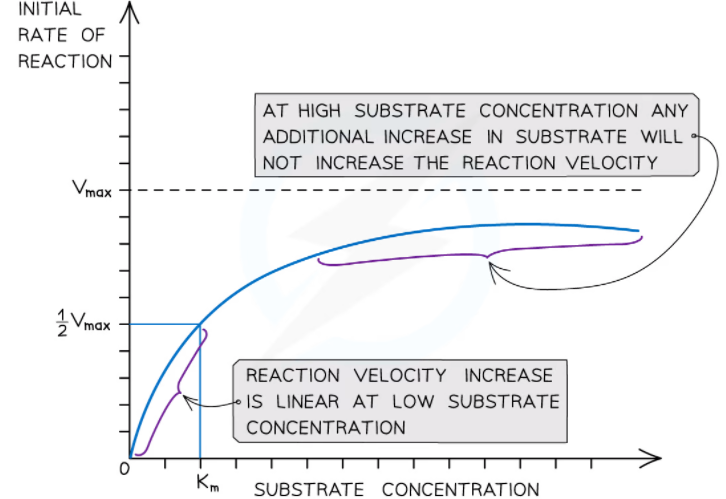
The kinetics of such enzyme-catalyzed reactions are explained by the Michaelis-Menten model. This model employs two values: maximal rate or maximal velocity (Vmax) and the Michaelis-Menten constant (Km) to describe an enzyme-catalyzed reaction. We can derive these values from the rate of reaction at various concentrations of the substrate. Maximal rate or maximal velocity (Vmax) and the Michaelis-Menten constant (Km) are derived from the maximum rate of reaction (Vmax), which is employed to compare the affinity of various enzymes for their substrates.
Kinetics of Michaelis-Menten enzyme
The two crucial deduced values are Vmax which is the highest reaction rate at saturating substrate concentrations and the Km, which is the substrate concentration at ½Vmax, also referred to as the Michaelis-Menten constant. The concentration of substrate at which the enzyme works at half its highest rate is known as the Michaelis-Menten constant.
At this point, the substrate molecules occupy half of the enzyme’s active sites. A lower concentration of the substrate will be required for this to occur if the affinity of the enzyme for the substrate is higher. Due to this reason, we say that the Michaelis-Menten constant measures the affinity of the enzyme for its substrate.
Km and the affinity of an enzyme have an inverse relationship between them. An enzyme has a high affinity for its substrate if there is high Km. Similarly, an enzyme has a low affinity for its substrate if there is a low Km.
Summarise with AI:













Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead