Chapters
In this article, we will discuss the effects of reversible inhibitors on enzyme activity. But before proceeding to discuss how reversible inhibitors affect the enzyme action, first, let us recall what are enzymes.

What are Enzymes?
Enzymes refer to the biological catalysts that speed up or catalyze the reactions occurring inside our bodies. Enzymes are also globular proteins as they are composed of large protein molecules. They generally catalyze the chemical reactions occurring inside our cells. An active site of an enzyme is critical for its function because it is the place where the substrate binds.
Importance of Enzymes
Enzymes maneuver metabolic pathways in a biochemical cascade of reactions. Nearly every metabolic reaction occurring inside the living organisms is sped up by an enzyme, hence enzymes are critical for the existence of life.
Enzymes can be intracellular or extracellular.
- Intracellular enzymes: These enzymes are produced and they function inside the cell
- Extracellular enzymes: Cells secrete these enzymes and they speed up the reactions outside the cells. For example, the digestive enzyme in the gut.
Factors that Affect the Enzyme’s Action
Many factors affect the enzyme’s action. These factors include enzyme concentration, substrate concentration, temperature, pH (hydrogen ion concentration), and the presence of inhibitors.
How do temperature and pH affect the enzyme’s action?
Since enzymes are composed of proteins, hence they are very sensitive to the changes in temperature. Enzymes function within a narrow range of temperatures. Each enzyme requires a specific temperature to function properly. This temperature is referred to as optimal temperature which ranges from 37oC to 40oC.
With the increase in temperature beyond the optimal temperature, the activity of the enzyme gradually slows down until the temperature increases to a point where the activity of the enzyme stops altogether.
A temperature lower than the optimal temperature also affects enzyme activity. If the temperature falls below the optimal temperature, the enzyme activity declines until the temperature reaches the point where the enzyme activity is minimum. At 0oC, the enzyme activity stops altogether. However, as the temperature rises, the enzyme activity starts again.
pH also controls the enzyme’s action. With the increase or decrease of pH, there is a change in the nature of different acid and amine groups on the side chains which result in alterations in the overall shape and structure of the enzyme.
Each enzyme has a specific pH value that allows it to work with the highest efficiency. This pH is referred to as optimal Ph. If the pH is higher or lower than the optimal pH, then the activity of the enzyme reduces until it stops working completely. For instance, pepsin works best at low pH because it is very acidic, whereas trypsin works best at high pH as it is basic. The optimal pH of the majority of the enzymes is 7.4.
Now, let us see what are enzyme inhibitors.
What are Enzyme Inhibitors?
Enzyme inhibitors refer to the molecules that interact with an enzyme to stop it from working in a normal way
There are several types of inhibitors which include non-specific, reversible, irreversible, competitive, and non-competitive. Examples of enzyme inhibitors include drugs and poisons.
In the next section of the article, we will discuss the reversible enzyme inhibitors.
Reversible Enzyme Inhibitors
A reversible inhibitor can slow down or stop an enzyme’s action. Reversible inhibitors are of two types:
- Competitive inhibitors: The shape of these inhibitors is the same as that of the substrate molecules, hence they compete with the substrate for the active site.
- Non-competitive inhibitors: These inhibitors bind to the enzyme at an alternative site which changes the shape of the active site and hence stops the substrate from binding to it.
- In metabolic pathways, reversible inhibitors can act as regulators. There should be a strict check and balance on metabolic reactions so that no enzyme can continuously and uncontrollably produce more and more of a specific product.
- The end-product of a specific sequence of metabolic reactions as a non-competitive, reversible inhibitor can control metabolic reactions.
- When the enzyme transforms substrate to product, the process itself slows down because the end product of the reaction chain binds to an alternative site on the original enzyme which alters the shape of the active site and stops the creation of more enzyme-substrate complexes.
- The end product can then disengage from the enzyme and can be employed anywhere else, thus enabling the active site to reform and helping the enzyme to return to an active state.
- This implies that when the product level declines, the enzyme starts to speed up the reaction once again in a non-stop feedback loop. This process is referred to as end-product inhibition.
Enzyme Activity: Immobilized vs Free
- Enzymes can be added to solutions and as a result, they are considered free or can be immobilized.
- Immobilized enzymes refer to the enzymes that are bound to an inert, stationary, and insoluble material like alginate
- After that, the substrate is passed over the immobilized enzyme and the product is accumulated.
Advantages
Advantages of this procedure include:
- The product is uncontaminated (there is no enzyme in the product) and hence there is no need to process or filter the end product further.
- The enzyme that is immobilized can be reused many times which is both efficient and cost-effective (because enzymes are costly). Immobilized enzymes have more tolerance to temperature and changes in pH. This is because immobilization makes enzymes more stable.
Practical Application of Immobilized Enzyme
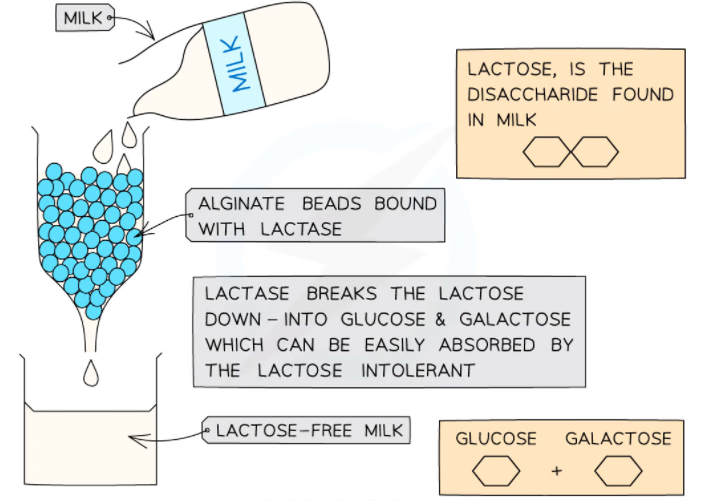
Immobilized enzymes are employed in the food industry to produce lactose-free milk. Milk is the natural source of essential nutrients such as fats, protein, and carbohydrate lactose. Almost 5 -10% of the population in the US is lactose intolerant. The disaccharide lactose can be broken down into galactose and glucose.
Lactase can be used to remove lactose from milk. In this way, individuals suffering from lactose intolerance can consume milk without any issue.
- By employing alginate beads, the enzyme lactase can be immobilized.
- Milk is passed through a column of beads containing lactase
- The lactose in the milk is hydrolyzed by lactase to galactose and glucose
- The entire process ensures that the milk is free from lactose
- This process can also be employed to produce other dairy products that are free from lactose.
Summarise with AI:













Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead