Chapters
In this article, we will discuss what are soluble and insoluble proteins. We will specifically discuss the globular proteins as soluble proteins and fibrous proteins as insoluble ones. Besides this, we will also discuss the molecular structures of haemoglobin and collagen in detail.

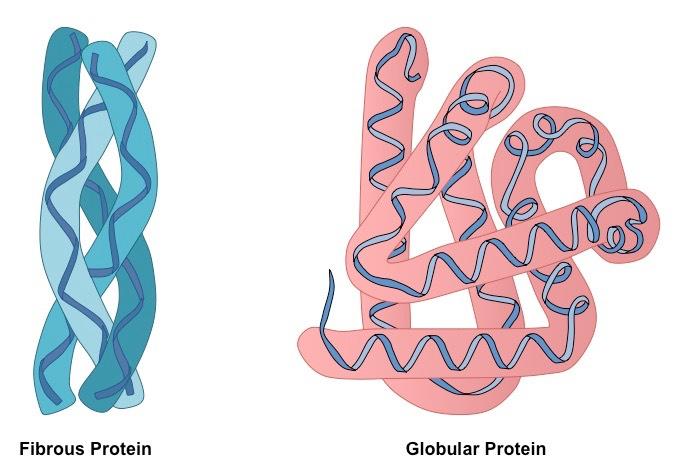
Globular and Fibrous Proteins
Globular Proteins
These proteins have a rough spherical (circular) shape and are compact. Globular proteins are soluble in water and form a spherical shape while folding into their tertiary structure because of the following reasons:
- The orientation of their non-polar hydrophobic R groups towards the centre of the protein away from the aqueous surroundings
- The orientation of their non-polar hydrophilic R groups on the exterior of the protein
Due to this orientation, globular proteins are generally soluble in water because the water molecules are able to surround the polar hydrophilic R groups. The solubility of globular proteins in water allows them to play crucial physiological roles because they can be moved easily around organisms and can participate in metabolic reactions.
The interactions between the R groups result in the folding of proteins. The specific shapes of the globular proteins are attributed to these foldings. They also help the globular proteins to play critical physiological roles, for instance, enzymes can speed up (catalyze) certain reactions and immunoglobulins can respond to certain antigens. Few globular proteins are conjugated proteins that have a prosthetic group. For example, haemoglobin (Hb) that has the prosthetic group is referred to as haem.
In the next section of the article, we will discuss fibrous proteins.
Fibrous Proteins
Fibrous proteins refer to the long strands of polypeptide chains that contain cross-linkages. The cross-linkages in fibrous proteins are attributed to the hydrogen bonds. These proteins contain little or no tertiary structure.
Fibrous proteins are insoluble in water because of the presence of a huge number of hydrophobic R groups in them. These proteins are characterized by the presence of a limited number of amino acids with sequence. However, the sequence of amino acids is highly repetitive in these proteins. The highly repetitive sequence forms extremely organized structures that are characterized by their strength and water insolubility. These two attributes make these proteins ideal for structural roles. For instance, keratin makes up nails, hair, horns, and feathers. On the other hand, collagen which is a connective tissue is present in tendons, ligaments, and skin.
In the next section of the article, we will discuss the molecular structure of haemoglobin.
Molecular Structure of Hemoglobin
Haemoglobin is an example of a globular protein. It is a pigment that is present in huge quantities in red blood cells and it carries oxygen.
Because it has four polypeptide chains or subunits, hence it has a quaternary structure. These chains are globin proteins, i.e. two α–globins and two β–globins. Each chain contains a prosthetic haem group.
Disulphide bonds hold up the four globin chains together and they are arranged so that their hydrophobic R groups face inwards and the hydrophilic R groups point outwards. The inwards facing of the hydrophobic R groups helps to maintain the three-dimensional spherical shape, whereas the outward-facing of the hydrophilic R groups helps to maintain solubility.
The functioning of haemoglobin depends on the arrangements of the R groups. Therefore, any variation in the sequence of amino acids in the chains can result in a change in the properties of haemoglobin. This nature of haemoglobin is attributed to the cause of sickle cell anaemia. In sickle cell anaemia, the base substitution causes the replacement of glutamic acid by amino acid valine, making haemoglobin less soluble.
The prosthetic haem group has an iron II which can reversibly combine with an oxygen molecule to produce oxyhemoglobin. This causes haemoglobin to appear bright red. Each haemoglobin with four haem groups can carry eight oxygen atoms (four oxygen molecules).
Functions of Haemoglobin
- Haemoglobin binds oxygen in the lung and carries oxygen to tissue to be employed in aerobic metabolic pathways.
- Because oxygen is less soluble in water as compared to haemoglobin, hence oxygen transports around the body more efficiently when it is bound to haemoglobin.
- The haem group in haemoglobin helps tiny molecules such as oxygen to be bound more efficiently. This is due to the fact that as each oxygen molecule binds, it changes the quaternary structure of proteins. This change in quaternary structure causes haemoglobin to have a greater affinity for the next oxygen molecules and they bind more easily.
- The presence of iron II in the prosthetic haem group also enables oxygen to bind reversibly because no amino acids that make up the polypeptide chains in haemoglobin are suitable to bind with oxygen.
The Molecular Structure of Collagen
Collagen is an insoluble fibrous protein that is widely found in structural proteins in vertebrates. It is the part of the connective tissue in vertebrates and forms cartilage, ligaments, tendons, teeth, bones, skin, walls of blood vessels, and the cornea of the eye.
Three polypeptide chains are held together closely by hydrogen bonds to create a triple helix form the collagen. Each helix-shaped polypeptide chain has almost 1000 amino acids with glycine, proline, and hydroxyproline.
Almost every third amino acid is glycine in the primary structure of this protein. Glycine is the smallest amino acid with an R group containing one hydrogen atom. Glycine is present in the inside of the polypeptide chains enabling three chains to be arranged closely together to create a tight triple helix structure.
Besides hydrogen bonds between the three chains, covalent bonds are also present which create cross-links between R groups in interacting triple helices when they are arranged parallel to each other. The collagen molecules are held together to form fibrils by these cross-links. The position of collagen molecules in the fibrils gives them staggered ends. Collagen fibres are created when several fibrils are arranged together. The positioning of collagen fibres allows them to be lined up with the forces they are resisting.
Function of Collagen
- Collagen is a flexible structural protein that creates connective tissues
- Due to the presence of several hydrogen bonds within the triple helix structure of collagen, it has great tensile strength. The tensile strength of the collagen helps it to resist large pulling forces without breaking or stretching.
- The staggered edges of the collagen molecules within the fibrils give them strength
- The high proportion of proline and hydroxyproline make collagen a stable protein.
- The length of the collagen molecules implies that they take a lot of time to dissolve in water. Hence, the collagen molecules are insoluble in water.
Summarise with AI:













Keep on teaching us,you are excellent teachers
This is great
Thanks a lot for this book,it really helped me a lot
It’s useful to me
Thanks a lot for your Better book!
It’s a perfect article, go ahead